Abstract
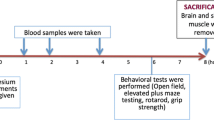
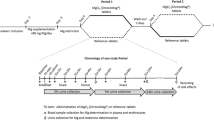
Magnesium, one of the basic elements for the human body, is necessary for many physiological functions. Magnesium deficiency is widely observed as a result of the reduced nutrient content of foods, over-cooking, diseases, drugs, alcohol, and caffeine consumption. Taking a dietary supplement is necessary magnesium deficiency. It has been demonstrated that absorption of organic magnesium compounds is better than absorption of inorganic compounds. The aim of this study is to investigate transitions to tissues of different organic magnesium compounds in different doses and whether there is a difference in the organic acid–bounded compounds (magnesium citrate and magnesium malate) and the amino acid–bounded compounds (magnesium acetyl taurate and magnesium glycinate), associated with transition and bioavailability. In addition, the effects of split dosages of high doses in a high volume of solvent on tissue magnesium levels are being investigated, because galenic formulation problems are regarded to prepare convenient dosage that can be taken once a day. All magnesium compounds were administered as three different doses, 45, 135, and 405 mg/70 kg elemental magnesium, were given per orally to Balbc mice. In a second set of experiments, 405 mg/70 kg high dose was divided into two doses of 202.5 mg/70 kg each and administered every 12 h. Brain, muscle tissues, and serum magnesium levels measured in all experimental groups and control 24 h later. Brain magnesium levels were found increased in all magnesium acetyl taurate administered subjects. Magnesium citrate increased muscle and brain magnesium levels in a dose-independent manner. We showed that dividing high doses of daily administered magnesium compounds did not sufficiently increase tissue magnesium levels. Although passive paracellular mechanism by solvent drag is the main mechanism of Mg absorption, other factors (electrochemical gradient effects, transcellular transporter mechanisms, magnesium status) should be effective on our results. It is necessary for further research on long-term administration of different magnesium compounds and their effect on other tissues.






Similar content being viewed by others
References
Jiafu SZGYY (1995) Effect of organic manure on qualities of crops. Plant Nutrition and Fertilizing Science 2:007
DiNicolantonio JJ, O’Keefe JH (2018) Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Heart 5(1):e000668. https://doi.org/10.1136/openhrt-2017-000668
Swaminathan R (2003) Magnesium metabolism and its disorders. Clin Biochem Rev 24(2):47–66
Naithani M, Bharadwaj J, Darbari A (2014) Magnesium: the fifth electrolyte. Journal of Medical Nutrition and Nutraceuticals 3(2):66
Noronha JL, Matuschak GM (2002) Magnesium in critical illness: metabolism, assessment, and treatment. Intensive Care Med 28(6):667–679. https://doi.org/10.1007/s00134-002-1281-y
de Baaij JH, Hoenderop JG, Bindels RJ (2015) Magnesium in man: implications for health and disease. Physiol Rev 95(1):1–46. https://doi.org/10.1152/physrev.00012.2014
Schuchardt JP, Hahn A (2017) Intestinal absorption and factors influencing bioavailability of magnesium—an update. Curr Nutr Food Sci 13(4):260–278. https://doi.org/10.2174/1573401313666170427162740
Uysal N, Kizildag S, Yuce Z, Guvendi G, Kandis S, Koc B, Karakilic A, Camsari UM, Ates M (2018) Timeline (bioavailability) of magnesium compounds in hours: which magnesium compound works best? Biol Trace Elem Res 187:128–136. https://doi.org/10.1007/s12011-018-1351-9
Coudray C, Rambeau M, Feillet-Coudray C, Gueux E, Tressol JC, Mazur A, Rayssiguier Y (2005) Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnes Res 18(4):215–223
Fine KD, Santa Ana CA, Porter JL, Fordtran JS (1991) Intestinal absorption of magnesium from food and supplements. J Clin Invest 88(2):396–402. https://doi.org/10.1172/jci115317
Karagulle O, Kleczka T, Vidal C, Candir F, Gundermann G, Kulpmann WR, Gehrke A, Gutenbrunner C (2006) Magnesium absorption from mineral waters of different magnesium content in healthy subjects. Forsch Komplementarmed 13(1):9–14. https://doi.org/10.1159/000090016
Musso CG (2009) Magnesium metabolism in health and disease. Int Urol Nephrol 41(2):357–362
Nishizawa Y, Morii H, Durlach J (2007) New perspectives in magnesium research. Springer, Berlin
Jahnen-Dechent W, Ketteler M (2012) Magnesium basics. Clin Kidney J 5(Suppl 1):i3–i14. https://doi.org/10.1093/ndtplus/sfr163
Ranade VV, Somberg JC (2001) Bioavailability and pharmacokinetics of magnesium after administration of magnesium salts to humans. Am J Ther 8(5):345–357
Hartle JW, Morgan S, Poulsen T (2016) Development of a model for in-vitro comparative absorption of magnesium from five magnesium sources commonly used as dietary supplements. FASEB J 30(1_supplement):128.126
Razak MA, Begum PS, Viswanath B, Rajagopal S (2017) Multifarious beneficial effect of nonessential amino acid, glycine: a review. Oxidative Med Cell Longev 2017:1–8
Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ (2003) L-glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr & Metab Care 6(2):229–240
Larson MD (1983) Glycine and the blood-brain barrier. Anesthesiology 58(5):488–489
Kang YS, Ohtsuki S, Takanaga H, Tomi M, Hosoya K, Terasaki T (2002) Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-alpha, taurine and hypertonicity. J Neurochem 83(5):1188–1195
Kang YS (2000) Taurine transport mechanism through the blood-brain barrier in spontaneously hypertensive rats. Adv Exp Med Biol 483:321–324
Kong WX, Chen SW, Li YL, Zhang YJ, Wang R, Min L, Mi X (2006) Effects of taurine on rat behaviors in three anxiety models. Pharmacol Biochem Behav 83(2):271–276. https://doi.org/10.1016/j.pbb.2006.02.007
Tamai I, Senmaru M, Terasaki T, Tsuji A (1995) Na(+)- and Cl(-)-dependent transport of taurine at the blood-brain barrier. Biochem Pharmacol 50(11):1783–1793
Tsuji A, Tamai I (1996) Sodium- and chloride-dependent transport of taurine at the blood-brain barrier. Adv Exp Med Biol 403:385–391
Konishi M (2005) Cell membrane transport of magnesium. Clin calcium 15(2):233–238
Beyenbach KW (1990) Transport of magnesium across biological membranes. Magnes Trace Elem 9(5):233–254
Gnoni GV, Priore P, Geelen MJ, Siculella L (2009) The mitochondrial citrate carrier: metabolic role and regulation of its activity and expression. IUBMB Life 61(10):987–994. https://doi.org/10.1002/iub.249
Bhutia YD, Kopel JJ, Lawrence JJ, Neugebauer V, Ganapathy V (2017) Plasma membrane Na(+)-coupled citrate transporter (SLC13A5) and neonatal epileptic encephalopathy. Molecules 22(3). https://doi.org/10.3390/molecules22030378
Chiu HY, Yeh TH, Huang YC, Chen PY (2016) Effects of intravenous and oral magnesium on reducing migraine: a meta-analysis of randomized controlled trials. Pain Physician 19(1):E97–E112
Dahle LO, Berg G, Hammar M, Hurtig M, Larsson L (1995) The effect of oral magnesium substitution on pregnancy-induced leg cramps. Am J Obstet Gynecol 173(1):175–180
Roffe C, Sills S, Crome P, Jones P (2002) Randomised, cross-over, placebo controlled trial of magnesium citrate in the treatment of chronic persistent leg cramps. Med Sci Monit 8(5):Cr326–Cr330
Garrison SR, Allan GM, Sekhon RK, Musini VM, Khan KM (2012) Magnesium for skeletal muscle cramps. Cochrane Database Syst Rev (9):Cd009402. https://doi.org/10.1002/14651858.CD009402.pub2
Nygaard IH, Valbo A, Pethick SV, Bohmer T (2008) Does oral magnesium substitution relieve pregnancy-induced leg cramps? Eur J Obstet Gynecol Reprod Biol 141(1):23–26. https://doi.org/10.1016/j.ejogrb.2008.07.005
Zhou K, West HM, Zhang J, Xu L, Li W (2015) Interventions for leg cramps in pregnancy. Cochrane Database Syst Rev (8):Cd010655. https://doi.org/10.1002/14651858.CD010655.pub2
Dai Z, Zhou H, Zhang S, Gu H, Yang Q, Zhang W, Dong W, Ma J, Fang Y, Jiang M, Xin F (2018) Current advance in biological production of malic acid using wild type and metabolic engineered strains. Bioresour Technol 258:345–353. https://doi.org/10.1016/j.biortech.2018.03.001
Huypens P, Pillai R, Sheinin T, Schaefer S, Huang M, Odegaard ML, Ronnebaum SM, Wettig SD, Joseph JW (2011) The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia 54(1):135–145. https://doi.org/10.1007/s00125-010-1923-5
Verhas M, de la Gueronniere V, Grognet JM, Paternot J, Hermanne A, Van den Winkel P, Gheldof R, Martin P, Fantino M, Rayssiguier Y (2002) Magnesium bioavailability from mineral water. A study in adult men. Eur J Clin Nutr 56(5):442–447. https://doi.org/10.1038/sj.ejcn.1601333
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments was carried out according to the Guiding Principles in the Use of Experimental Animals and approved by the Animal Care and Use Committee of the Dokuz Eylul University, School of Medicine.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ates, M., Kizildag, S., Yuksel, O. et al. Dose-Dependent Absorption Profile of Different Magnesium Compounds. Biol Trace Elem Res 192, 244–251 (2019). https://doi.org/10.1007/s12011-019-01663-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01663-0