Abstract
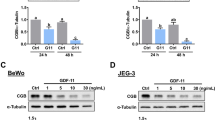
Selenoprotein K (SelK), a member of selenoprotein family, is identified as a single endoplasmic reticulum (ER) transmembrane protein. Although over-expression of SelK inhibits adherence and migration of human gastric cancer BGC-823 cells, the effects of SelK in human choriocarcinoma (CCA) are not well understood. In this study, the expression levels of SelK in three CCA cell lines, BeWo, JEG-3, and JAR, were examined. The effects of silencing or over-expressing SelK on expression of human chorionic gonadotropin beta subunit (β-hCG) were detected by western blotting. The results show that the protein level of β-hCG was reciprocally regulated by down- or up-regulation of SelK (*P < 0.05; #P < 0.05). The proliferative, migratory, and invasive capabilities of JEG-3 cells with reduced or over-expressed SelK were then tested using the cell counting kit-8 (CCK-8), wound healing, and transwell chamber assays. We found that these cellular activities were markedly increased by the loss of SelK in JEG-3 cells. Conversely, over-expressing SelK in JEG-3 cells suppressed these phenotypes. In addition, SelK expression after down- or up-regulation of β-hCG was also measured. Surprisingly, we found that level of SelK was affected by β-hCG (*P < 0.05; #P < 0.05). The proliferation, migration, and invasion were determined in JEG-3 cells after each over-expression and reduction of β-hCG. The results confirmed that β-hCG functions as a promoter of human choriocarcinoma. Furthermore, ERK/p38 MAPK and Akt signaling pathways were found to involve in these cellular functions. This work suggests that SelK may act as a tumor suppressor in human choriocarcinoma cells by negatively regulating β-hCG expression via ERK, p38 MAPK, and Akt signaling pathways. These findings revealed that selenoprotein K may serve as a novel target for human choriocarcinoma therapy in vitro.






Similar content being viewed by others
References
Seckl MJ, Sebire NJ, Berkowitz RS (2010) Gestational trophoblastic disease. Lancet (London, England) 376(9742):717–729. https://doi.org/10.1016/s0140-6736(10)60280-2
Ho J, Du Y, Wong OG, Siu MK, Chan KK, Cheung AN (2014) Downregulation of the gli transcription factors regulator Kif7 facilitates cell survival and migration of choriocarcinoma cells. PLoS One 9(9):e108248. https://doi.org/10.1371/journal.pone.0108248
Shih Ie M (2007) Gestational trophoblastic neoplasia––pathogenesis and potential therapeutic targets. Lancet Oncol 8(7):642–650. https://doi.org/10.1016/s1470-2045(07)70204-8
Lewis JL Jr (1993) Diagnosis and management of gestational trophoblastic disease. Cancer 71(4 Suppl):1639–1647
Cheung AN (2003) Pathology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol 17(6):849–868
Yang J, Xiang Y, Wan X, Yang X (2006) Recurrent gestational trophoblastic tumor: management and risk factors for recurrence. Gynecol Oncol 103(2):587–590. https://doi.org/10.1016/j.ygyno.2006.04.007
Berkowitz RS, Goldstein DP (2013) Current advances in the management of gestational trophoblastic disease. Gynecol Oncol 128(1):3–5. https://doi.org/10.1016/j.ygyno.2012.07.116
Vinceti M, Dennert G, Crespi CM, Zwahlen M, Brinkman M, Zeegers MP, Horneber M, D'Amico R, Del Giovane C (2014) Selenium for preventing cancer. Cochrane Database Syst Rev (3):Cd005195. https://doi.org/10.1002/14651858.CD005195.pub3
Shamberger RJ, Frost DV (1969) Possible protective effect of selenium against human cancer. Can Med Assoc J 100(14):682
Schrauzer GN, White DA, Schneider CJ (1977) Cancer mortality correlation studies––III: statistical associations with dietary selenium intakes. Bioinorg Chem 7(1):23–31
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FLJ, Baker LH, Coltman CA,J (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama 301(1):39–51. https://doi.org/10.1001/jama.2008.864
Steinbrenner H, Speckmann B, Klotz LO (2016) Selenoproteins: antioxidant selenoenzymes and beyond. Arch Biochem Biophys 595:113–119. https://doi.org/10.1016/j.abb.2015.06.024
Saito Y, Takahashi K (2002) Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem 269(22):5746–5751. https://doi.org/10.1046/j.1432-1033.2002.03298.x
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9(7):775–806. https://doi.org/10.1089/ars.2007.1528
Pitts MW, Hoffmann PR (2017) Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium. https://doi.org/10.1016/j.ceca.2017.05.001
Ellgaard L, McCaul N, Chatsisvili A, Braakman I (2016) Co- and post-translational protein folding in the ER. Traffic (Copenhagen, Denmark) 17(6):615–638. https://doi.org/10.1111/tra.12392
Morozova N, Forry EP, Shahid E, Zavacki AM, Harney JW, Kraytsberg Y, Berry MJ (2003) Antioxidant function of a novel selenoprotein in Drosophila melanogaster. Genes Cells: Devoted Mol Cell Mech 8(12):963–971
Ben SB, Wang QY, Xia L, Xia JZ, Cui J, Wang J, Yang F, Bai H, Shim MS, Lee BJ, Sun LG, Chen CL (2011) Selenoprotein dSelK in Drosophila elevates release of Ca2+ from endoplasmic reticulum by upregulating expression of inositol 1,4,5-tris-phosphate receptor. Biochem Biokhim 76(9):1030–1036. https://doi.org/10.1134/S0006297911090070
Fredericks GJ, Hoffmann PR (2015) Selenoprotein K and protein palmitoylation. Antioxid Redox Signal 23(10):854–862. https://doi.org/10.1089/ars.2015.6375
Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR (2011) Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol (Baltimore, Md : 1950) 186(4):2127–2137. https://doi.org/10.4049/jimmunol.1002878
Du S, Zhou J, Jia Y, Huang K (2010) SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys 502(2):137–143. https://doi.org/10.1016/j.abb.2010.08.001
Shchedrina VA, Everley RA, Zhang Y, Gygi SP, Hatfield DL, Gladyshev VN (2011) Selenoprotein K binds multiprotein complexes and is involved in the regulation of endoplasmic reticulum homeostasis. J Biol Chem 286(50):42937–42948. https://doi.org/10.1074/jbc.M111.310920
Lee JH, Park KJ, Jang JK, Jeon YH, Ko KY, Kwon JH, Lee SR, Kim IY (2015) Selenoprotein S-dependent selenoprotein K binding to p97(VCP) protein is essential for endoplasmic reticulum-associated degradation. J Biol Chem 290(50):29941–29952. https://doi.org/10.1074/jbc.M115.680215
Ben SB, Peng B, Wang GC, Li C, Gu HF, Jiang H, Meng XL, Lee BJ, Chen CL (2015) Overexpression of selenoprotein SelK in BGC-823 cells inhibits cell adhesion and migration. Biochem Biokhim 80(10):1344–1353. https://doi.org/10.1134/s0006297915100168
Meplan C, Rohrmann S, Steinbrecher A, Schomburg L, Jansen E, Linseisen J, Hesketh J (2012) Polymorphisms in thioredoxin reductase and selenoprotein K genes and selenium status modulate risk of prostate cancer. PLoS One 7(11):e48709. https://doi.org/10.1371/journal.pone.0048709
Guariniello S, Di Bernardo G, Colonna G, Cammarota M, Castello G, Costantini S (2015) Evaluation of the selenotranscriptome expression in two hepatocellular carcinoma cell lines. Anal Cell Pathol (Amsterdam) 2015:419561. https://doi.org/10.1155/2015/419561
Ozalp SS, Oge T (2013) Gestational trophoblastic diseases in Turkey. J Reprod Med 58(1–2):67–71
Stevens FT, Katzorke N, Tempfer C, Kreimer U, Bizjak GI, Fleisch MC, Fehm TN (2015) Gestational trophoblastic disorders: an update in 2015. Geburtshilfe Frauenheilkd 75(10):1043–1050. https://doi.org/10.1055/s-0035-1558054
Bhatti P, Stewart PA, Hutchinson A, Rothman N, Linet MS, Inskip PD, Rajaraman P (2009) Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol, Biomarkers Prev: Publ Am Assoc Cancer Res, Cosponsored Am Soc Prev Oncology 18(6):1841–1848. https://doi.org/10.1158/1055-9965.epi-09-0197
Dokic I, Hartmann C, Herold-Mende C, Regnier-Vigouroux A (2012) Glutathione peroxidase 1 activity dictates the sensitivity of glioblastoma cells to oxidative stress. Glia 60(11):1785–1800. https://doi.org/10.1002/glia.22397
Pohl NM, Tong C, Fang W, Bi X, Li T, Yang W (2009) Transcriptional regulation and biological functions of selenium-binding protein 1 in colorectal cancer in vitro and in nude mouse xenografts. PLoS One 4(11):e7774. https://doi.org/10.1371/journal.pone.0007774
de Sousa AR, Penalva LO, Marcotte EM, Vogel C (2009) Global signatures of protein and mRNA expression levels. Mol BioSyst 5(12):1512–1526. https://doi.org/10.1039/b908315d
Lu C, Qiu F, Zhou H, Peng Y, Hao W, Xu J, Yuan J, Wang S, Qiang B, Xu C, Peng X (2006) Identification and characterization of selenoprotein K: an antioxidant in cardiomyocytes. FEBS Lett 580(22):5189–5197. https://doi.org/10.1016/j.febslet.2006.08.065
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443. https://doi.org/10.1126/science.1083516
Liu J, Srinivasan P, Pham DN, Rozovsky S (2012) Expression and purification of the membrane enzyme selenoprotein K. Protein Expr Purif 86(1):27–34. https://doi.org/10.1016/j.pep.2012.08.014
Shchedrina VA, Zhang Y, Labunskyy VM, Hatfield DL, Gladyshev VN (2010) Structure-function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antioxid Redox Signal 12(7):839–849. https://doi.org/10.1089/ars.2009.2865
Lee JH, Kwon JH, Jeon YH, Ko KY, Lee SR, Kim IY (2014) Pro178 and Pro183 of selenoprotein S are essential residues for interaction with p97(VCP) during endoplasmic reticulum-associated degradation. J Biol Chem 289(20):13758–13768. https://doi.org/10.1074/jbc.M113.534529
Guo X, Liu G, Schauer IG, Yang G, Mercado-Uribe I, Yang F, Zhang S, He Y, Liu J (2011) Overexpression of the beta subunit of human chorionic gonadotropin promotes the transformation of human ovarian epithelial cells and ovarian tumorigenesis. Am J Pathol 179(3):1385–1393. https://doi.org/10.1016/j.ajpath.2011.05.018
Fournier T, Guibourdenche J, Evain-Brion D (2015) Review: hCGs: different sources of production, different glycoforms and functions. Placenta 36(Suppl 1):S60–S65. https://doi.org/10.1016/j.placenta.2015.02.002
Montagnana M, Trenti T, Aloe R, Cervellin G, Lippi G (2011) Human chorionic gonadotropin in pregnancy diagnostics. Clin Chim Acta; Int J Clin Chemistry 412(17–18):1515–1520. https://doi.org/10.1016/j.cca.2011.05.025
Cole LA, Khanlian SA, Muller CY, Giddings A, Kohorn E, Berkowitz R (2006) Gestational trophoblastic diseases: 3. Human chorionic gonadotropin-free beta-subunit, a reliable marker of placental site trophoblastic tumors. Gynecol Oncol 102(2):160–164. https://doi.org/10.1016/j.ygyno.2005.12.046
Cole LA (2012) hCG, the wonder of today’s science. Reprod Biol Endocrinol: RB&E 10:24. https://doi.org/10.1186/1477-7827-10-24
He LZ, Ramakrishna V, Connolly JE, Wang XT, Smith PA, Jones CL, Valkova-Valchanova M, Arunakumari A, Treml JF, Goldstein J, Wallace PK, Keler T, Endres MJ (2004) A novel human cancer vaccine elicits cellular responses to the tumor-associated antigen, human chorionic gonadotropin beta. Clin Cancer Res: Off J Am Assoc Cancer Res 10(6):1920–1927
Ruddon RW, Sherman SA, Bedows E (1996) Protein folding in the endoplasmic reticulum: lessons from the human chorionic gonadotropin beta subunit. Protein Sci: Publ Protein Soc 5(8):1443–1452. https://doi.org/10.1002/pro.5560050801
Sisinni L, Landriscina M (2015) The role of human chorionic gonadotropin as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol 867:159–176. https://doi.org/10.1007/978-94-017-7215-0_11
Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802(4):396–405. https://doi.org/10.1016/j.bbadis.2009.12.009
Burotto M, Chiou VL, Lee JM, Kohn EC (2014) The MAPK pathway across different malignancies: a new perspective. Cancer 120(22):3446–3456. https://doi.org/10.1002/cncr.28864
Nadeau V, Charron J (2014) Essential role of the ERK/MAPK pathway in blood-placental barrier formation. Development (Cambridge, England) 141(14):2825–2837. https://doi.org/10.1242/dev.107409
Yung HW, Charnock-Jones DS, Burton GJ (2011) Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One 6(3):e17894. https://doi.org/10.1371/journal.pone.0017894
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Selenoprotein K (SelK) acts as a tumor suppressor in choriocarcinoma cells.
• A reciprocally negative regulation exists between SelK and human chorionic gonadotropin (hCG) in choriocarcinoma cells.
• The ERK/p38 MAPK and Akt signaling pathways are involved in these pathologies.
Rights and permissions
About this article
Cite this article
Li, M., Cheng, W., Nie, T. et al. Selenoprotein K Mediates the Proliferation, Migration, and Invasion of Human Choriocarcinoma Cells by Negatively Regulating Human Chorionic Gonadotropin Expression via ERK, p38 MAPK, and Akt Signaling Pathway. Biol Trace Elem Res 184, 47–59 (2018). https://doi.org/10.1007/s12011-017-1155-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1155-3