Abstract
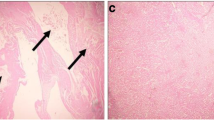
For experiments of cadmium toxicity in animal models, cadmium (II) chloride is often used due to its solubility in water and its ability to produce high concentrations of cadmium at the target site. The present study was designed to investigate the potential inhibitory effect of the Fragaria ananassa fruit extract on cadmium (II) chloride-induced renal toxicity in rats. Tested animals were pretreated with the extract of F. ananassa and injected with cadmium (II) chloride (6.5-mg/kg body weight) for 5 days. Cadmium (II) chloride significantly increased kidney cadmium concentration, kidney weight, lipid peroxidation, and nitric oxide production. Plasma uric acid, urea, and creatinine levels also increased significantly, indicative of kidney dysfunction. These effects were accompanied by significantly decreased levels of nonenzymatic and enzymatic antioxidant molecules (i.e., glutathione content and the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase). Moreover, messenger RNA (mRNA) expression of the antiapoptotic protein, Bcl-2, and the antioxidant proteins, superoxide dismutase 2 and glutathione reductase, were downregulated markedly, whereas mRNA expression of tumor necrosis factor-α was upregulated significantly in kidney tissues of cadmium-treated rats. Histology of kidney tissue demonstrated severe, adverse changes that reflected cadmium-induced tissue damage. Pretreatment of rats with the extract of F. ananassa ameliorated all aforementioned cadmium (II) chloride-induced changes. In conclusion, the present study showed acute renal toxicity in rats treated with cadmium (II) chloride. The study also revealed that pretreatment with the extract of F. ananassa could protect the kidney against cadmium (II) chloride-induced acute renal toxicity.








Similar content being viewed by others
References
Jarup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208
Satarug S, Nishijo M, Ujjin P, Vanavanitkun Y, Moore MR (2005) Cadmium-induced nephropathy in the development of high blood pressure. Toxicol Lett 157(1):57–68
Meyer KJ, Reif JS, Veeramachaneni DN, Luben TJ, Mosley BS, Nuckols JR (2006) Agricultural pesticide use and hypospadias in eastern Arkansas. Environ Health Perspect 114(10):1589–1595
Yang H, Shu Y (2015) Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci 16(1):1484–1494
Ansari MA, Raish M, Ahmad A, Alkharfy KM, Ahmad SF, Attia SM, Alsaad AM, Bakheet SA (2017) Sinapic acid ameliorate cadmium-induced nephrotoxicity: in vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-kappaB downregulation. Environ Toxicol Pharmacol
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health res 24(4):378–399. doi:10.1080/09603123.2013.835032
Dorian C, Gattone Ii VH, Klaasen CD (1992) Renal cadmium deposition and injury as a result of accumulation of cadmium-metallothionein (CdMT) by the proximal convoluted tubules—a light microscopic autoradiography study with 109CdMT. Toxicol Appl Pharmacol 114(2):173–181. doi:10.1016/0041-008X(92)90066-2
El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA (2007) Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 235(3):185–193
Aaby K, Skrede G, Wrolstad RE (2005) Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J Agric Food Chem 53(10):4032–4040. doi:10.1021/jf048001o
MilivojeviĆ J, MaksimoviĆ V, NikoliĆ M, BogdanoviĆ J, MaletiĆ R, MilatoviĆ D (2011) Chemical and antioxidant properties of cultivated and wild fragaria and rubus berries. J Food Qual 34(1):1–9. doi:10.1111/j.1745-4557.2010.00360.x
Hamed SS, Al-Yhya NA, El-Khadragy MF, Al-Olayan EM, Alajmi RA, Hassan ZK, Hassan SB, Abdel Moneim AE (2016) The protective properties of the strawberry (Fragaria ananassa) against carbon tetrachloride-induced hepatotoxicity in rats mediated by anti-apoptotic and upregulation of antioxidant genes expression effects. Front Physiol 7:325. doi:10.3389/fphys.2016.00325
Oszmiański J, Wojdyło A (2009) Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur Food res Technol 228(4):623–631. doi:10.1007/s00217-008-0971-2
Elkhadragy MF, Abdel Moneim AE (2017) Protective effect of Fragaria ananassa methanolic extract on cadmium chloride (CdCl2)-induced hepatotoxicity in rats. Toxicol Mech Methods:1–27. doi:10.1080/15376516.2017.1285973
Carlton PS, Kresty LA, Siglin JC, Morse MA, Lu J, Morgan C, Stoner GD (2001) Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis 22(3):441–446
Naemura A, Mitani T, Ijiri Y, Tamura Y, Yamashita T, Okimura M, Yamamoto J (2005) Anti-thrombotic effect of strawberries. Blood Coagul Fibrinolysis 16(7):501–509
Ibrahim DS, Abd El-Maksoud MA (2015) Effect of strawberry (Fragaria × ananassa) leaf extract on diabetic nephropathy in rats. Int J Exp Pathol 96(2):87–93. doi:10.1111/iep.12116
Abdel Moneim AE (2013) The neuroprotective effects of Purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurol Disord Drug Targets
Dkhil MA, Al-Quraishy S, Diab MM, Othman MS, Aref AM, Abdel Moneim AE (2014) The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem Toxicol 74:98–106
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J lab Clin med 70(1):158–169
De Vega L, Fernandez RP, Mateo MC, Bustamante JB, Herrero AM, Munguira EB (2002) Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail 24(4):421–432
Emamverdian A, Ding Y, Mokhberdoran F, Xie Y (2015) Heavy metal stress and some mechanisms of plant defense response. Sci World J 2015:18. doi:10.1155/2015/756120
Haouem S, El Hani A (2013) Effect of cadmium on lipid peroxidation and on some antioxidants in the liver, kidneys and testes of rats given diet containing cadmium-polluted radish bulbs. J Toxicol Pathol 26(4):359–364. doi:10.1293/tox.2013-0025
Chen J, Du L, Li J, Song H (2016) Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem Toxicol 96:70–78
Hwang DF, Wang LC (2001) Effect of taurine on toxicity of cadmium in rats. Toxicology 167(3):173–180
Pari L, Murugavel P (2005) Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ Toxicol Pharmacol 20(3):493–500
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013:162750. doi:10.1155/2013/162750
Kehrer JP, Klotz LO (2015) Free radicals and related reactive species as mediators of tissue injury and disease: implications for health. Crit rev Toxicol 45(9):765–798. doi:10.3109/10408444.2015.1074159
Reyes JL, Molina-Jijon E, Rodriguez-Munoz R, Bautista-Garcia P, Debray-Garcia Y, Namorado Mdel C (2013) Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed res Int 2013:730789. doi:10.1155/2013/730789
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651
Boora F, Chirisa E, Mukanganyama S (2014) Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J Food Process 2014:7. doi:10.1155/2014/918018
Pastore A, Federici G, Bertini E, Piemonte F (2003) Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 333(1):19–39
Bast A, Haenen GR (1988) Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim Biophys Acta 963(3):558–561
Schauder A, Avital A, Malik Z (2010) Regulation and gene expression of heme synthesis under heavy metal exposure—review. J Environ Pathol Toxicol Oncol 29(2):137–158
Adaramoye OA, Akanni OO (2016) Modulatory effects of methanol extract of Artocarpus altilis (Moraceae) on cadmium-induced hepatic and renal toxicity in male Wistar rats. Pathophysiology 23(1):1–9
Alghasham A, Salem TA, Meki AR (2013) Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-alpha, interleukin-6 and oxidative status biomarkers in rats: protective effect of curcumin. Food Chem Toxicol 59:160–164. doi:10.1016/j.fct.2013.05.059
Fouad AA, Jresat I (2015) Thymoquinone therapy abrogates toxic effect of cadmium on rat testes. Andrologia 47(4):417–426. doi:10.1111/and.12281
Stosic J, Mirkov I, Belij S, Nikolic M, Popov A, Kataranovski D, Kataranovski M (2010) Gender differences in pulmonary inflammation following systemic cadmium administration in rats. Biomed Environ Sci 23(4):293–299
Hagar H, Al Malki W (2014) Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: role of oxidative stress and caspase-3. Environ Toxicol Pharmacol 37(2):803–811
Al-Assaf AH, Alqahtani AM, Alshatwi AA, Syed NA, Shafi G, Hasan TN (2013) Mechanism of cadmium induced apoptosis in human peripheral blood lymphocytes: the role of p53, Fas and caspase-3. Environ Toxicol Pharmacol 36(3):1033–1039
Elmallah MIY, Elkhadragy MF, Al-Olayan EM, Abdel Moneim AE (2017) Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int J Mol Sci 18 (5)
Kondoh M, Araragi S, Sato K, Higashimoto M, Takiguchi M, Sato M (2002) Cadmium induces apoptosis partly via caspase-9 activation in HL-60 cells. Toxicology 170(1–2):111–117
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One 11(7):e0158965. doi:10.1371/journal.pone.0158965
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research through Research Group Project No. RG-1435-016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Elkhadragy, M.F., Al-Olayan, E.M., Al-Amiery, A.A. et al. Protective Effects of Fragaria ananassa Extract Against Cadmium Chloride-Induced Acute Renal Toxicity in Rats. Biol Trace Elem Res 181, 378–387 (2018). https://doi.org/10.1007/s12011-017-1062-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1062-7