Abstract
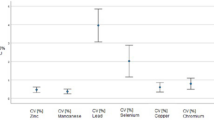
To better understand the relationship between prenatal exposure to heavy metals and trace elements and the risk of adverse pregnancy outcomes, we investigated the status of heavy metals and trace elements level in a Chinese population by collecting umbilical cord blood. Umbilical cord blood heavy metals and trace elements concentrations were determined by inductively coupled plasma–mass spectrometry. No differences with statistical significance in the median arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), manganese (Mn), nickel (Ni), lead (Pb), strontium (Sr), thallium (Tl), vanadium (V), and zinc (Zn) concentrations were observed between the adverse pregnancy outcome group and the reference group. Titanium (Ti) and antimony (Sb) were found at higher levels with statistical significance in the cord blood samples with adverse pregnancy group when compared to the ones in the reference group. The association between Ti levels and the risk of adverse pregnancy outcomes remained significant after adjusting for potential confounding factors, including newborn weight. These results indicated that environmental exposure to Ti may increase the risk of adverse pregnancy outcomes in Chinese women without occupational exposure.
Similar content being viewed by others
References
Al-Saleh I, Shinwari N, Mashhour A, Mohamed GD, Rabah A (2011) Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health 214(2):79–101
Xie X, Ding G, Cui C, Chen L, Gao Y, Zhou Y, Shi R, Tian Y (2013) The effects of low-level prenatal lead exposure on birth outcomes. Environ Pollut 175:30–34
Jin L, Zhang L, Li Z, Liu J, Ye R, Ren A (2013) Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reprod Toxicol 35:25–31
Liu X, Hu Q, Fang Z, Zhang X, Zhang B (2009) Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal. Langmuir 25(1):3–8
Sanyal A, Rautaray D, Bansal V, Ahmad A, Sastry M (2005) Heavy-metal remediation by a fungus as a means of production of lead and cadmium carbonate crystals. Langmuir 21(16):7220–7224
Wells PG, Lee CJ, McCallum GP, Perstin J, Harper PA (2010) Receptor-and reactive intermediate-mediated mechanisms of teratogenesis. In: Uetrecht J (ed) Adverse drug reactions, handbook of experimental pharmacology. Springer, Berlin, pp 131–162
Caserta D, Graziano A, Monte GL, Bordi G, Moscarini M (2013) Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur Rev Med Pharmacol Sci 17(16):2198–2206
Iyengar G, Rapp A (2001) Human placenta as a ‘dual’biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 3: toxic trace elements in placenta and placenta as a biomarker for these elements. Sci Total Environ 280(1):221–238
Cantonwine D, Hu H, Sánchez BN, Lamadrid-Figueroa H, Smith D, Ettinger AS, Mercado-García A, Hernández-Avila M, Wright RO, Téllez-Rojo MM (2010) Critical windows of fetal lead exposure: adverse impacts on length of gestation and risk of premature delivery. J Occup Environ Med 52(11):1106–1111
Ronco A, Urrutia M, Montenegro M, Llanos M (2009) Cadmium exposure during pregnancy reduces birth weight and increases maternal and foetal glucocorticoids. Toxicol Lett 188(3):186–191
Al-Saleh I, Shinwari N, Mashhour A, Rabah A (2013) Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int J Hyg Environ Health 217(2–3):205–218
Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, Bouige D, Lacroix C (2005) Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair: reference values. Forensic Sci Int 153(1):39–44
Butler Walker J, Houseman J, Seddon L, McMullen E, Tofflemire K, Mills C, Corriveau A, Weber JP, LeBlanc A, Walker M (2006) Maternal and umbilical cord blood levels of mercury, lead, cadmium, and essential trace elements in Arctic Canada. Environ Res 100(3):295–318
Parajuli RP, Fujiwara T, Umezaki M, Furusawa H, Ser PH, Watanabe C (2012) Cord blood levels of toxic and essential trace elements and their determinants in the Terai region of Nepal: a birth cohort study. Biol Trace Elem Res 147(1–3):75–83
Ni W, Huang Y, Wang X, Zhang J, Wu K (2014) Associations of neonatal lead, cadmium, chromium and nickel co-exposure with DNA oxidative damage in an electronic waste recycling town. Sci Total Environ 472:354–362
Ipach I, Schäfer R, Mittag F, Leichtle C, Wolf P, Kluba T (2012) The development of whole blood titanium levels after instrumented spinal fusion—is there a correlation between the number of fused segments and titanium levels? BMC Musculoskel Disord 13(1):159
Sicilia A, Cuesta S, Coma G, Arregui I, Guisasola C, Ruiz E, Maestro A (2008) Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. COIR 19(8):823–835
Arys A, Philippart C, Dourov N, He Y, Le Q, Pireaux JJ (1998) Analysis of titanium dental implants after failure of osseointegration: combined histological, electron microscopy, and X-ray photoelectron spectroscopy approach. J Biomed Mater Res 43(3):300–312
Cunningham BW, Orbegoso CM, Dmitriev AE, Hallab NJ, Sefter JC, McAfee PC (2002) The effect of titanium particulate on development and maintenance of a posterolateral spinal arthrodesis: an in vivo rabbit model. Spine 27(18):1971–1981
Li N, Duan Y, Hong M, Zheng L, Fei M, Zhao X, Wang J, Cui Y, Liu H, Cai J (2010) Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticules. Toxicol Lett 195(2):161–168
Chen J, Dong X, Zhao J, Tang G (2009) In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol 29(4):330–337
Wang Y, Chen Z, Ba T, Pu J, Chen T, Song Y, Gu Y, Qian Q, Xu Y, Xiang K (2013) Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 9(9–10):1742–1752
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69(22):8784–8789
Luo Z, Wang Z, Li Q, Pan Q, Yan C, Liu F (2011) Spatial distribution, electron microscopy analysis of titanium and its correlation to heavy metals: occurrence and sources of titanium nanomaterials in surface sediments from Xiamen Bay, China. J Environ Monit 13(4):1046–1052
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10(1):15
Liu H, Ma L, Zhao J, Liu J, Yan J, Ruan J, Hong F (2009) Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res 129(1–3):170–180
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168(2):176–185
Tamura K, Takashi N, Kumazawa R, Watari F, Totsuka Y (2002) Effects of particle size on cell function and morphology in titanium and nickel. Mater Trans 43(12):3052–3057
Bell ML, Belanger K, Ebisu K, Gent JF, Leaderer BP (2012) Relationship between birth weight and exposure to airborne fine particulate potassium and titanium during gestation. Environ Res 117:83–89
Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, Yoshida T, Ogura T, Nabeshi H, Nagano K, Abe Y, Kamada H, Monobe Y, Imazawa T, Aoshima H, Shishido K, Kawai Y, Mayumi T, Tsunoda S, Itoh N, Yoshikawa T, Yanagihara I, Saito S, Tsutsumi Y (2011) Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol 6(5):321–328
Xu L, Yu Y, Yu J, Chen J, Niu Z, Yin L, Zhang F, Liao X, Chen Y (2013) Spatial distribution and sources identification of elements in PM2.5among the coastal city group in the Western Taiwan Strait region, China. Sci Total Environ 442:77–85
Gao G, Ze Y, Li B, Zhao X, Zhang T, Sheng L, Hu R, Gui S, Sang X, Sun Q (2012) Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater 243:19–27
Zhao X, Ze Y, Gao G, Sang X, Li B, Gui S, Sheng L, Sun Q, Cheng J, Cheng Z (2013) Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS One 8(4):e59378
Acknowledgments
This work was supported by the project of the Xiamen Science and Technology Program (3502Z20114026) and the Fundamental Research Funds for the Central Universities (2012121045). We thank Dr. Yixin Yao for her assistance with the English language.
Conflict of Interest
The authors declared no conflicts of interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guanchao Zheng and Hongxiu Zhong are co-first authors.
Rights and permissions
About this article
Cite this article
Zheng, G., Zhong, H., Guo, Z. et al. Levels of Heavy Metals and Trace Elements in Umbilical Cord Blood and the Risk of Adverse Pregnancy Outcomes: a Population-Based Study. Biol Trace Elem Res 160, 437–444 (2014). https://doi.org/10.1007/s12011-014-0057-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0057-x