Abstract
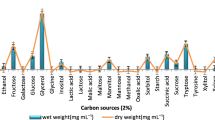
Bacterial cellulose (BC) is an emerging material for high-end applications due to its biocompatibility and physicochemical characteristics. However, the scale-up production of this material is still expensive, with the culture medium constituting one-third of the total cost. Herein, four different media (yeast nitrogen base, YNB; Murashige and Skoog, MSO; black tea; and NPK fertilizer solution) were compared while using sucrose as an additional carbon source. The yields of BC were best for YNB and fertilizer with 0.37 and 0.34 gBC/gC respectively. These two were then compared using glucose as a carbon source, with improvements in the production of 29% for the fertilizer, while only an 8% increase for YNB was seen; however, as the carbon concentration increased with a fixed N concentration, the yield was lower but the rate of production of BC increased. The obtained BC films were sanitized and showed low molecular weight and all the expected cellulose characteristic FT-IR bands while SEM showed nanofibers around 0.1 μm. Compared to traditional methods for lab-scale production, the use of the fertilizer and the consortium represent benefits compared to traditional lab-scale BC culture methods such as a competitive cost (two times lower) while posing resilience and tolerance to stress conditions given that it is produced by microbial communities and not with a single strain. Additionally, the low molecular weight of the films could be of interest for certain coating formulations.








Similar content being viewed by others
Data Availability
Data will be available on reasonable request.
References
Ullah, H., Wahid, F., Santos, H. A., & Khan, T. (2016). Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydrate Polymers, 150, 330–352. https://doi.org/10.1016/j.carbpol.2016.05.029.
Khajavi, R., Esfahani, E. J., & Sattari, M. (2011). Crystalline structure of microbial cellulose compared with native and regenerated cellulose. International Journal of Polymeric Materials, 60, 1178–1192. https://doi.org/10.1080/00914037.2010.551372
Gayathry, G. (2015). Production of nata de coco - a natural dietary fibre product from mature coconut water using Gluconacetobacter xylinum (sju-1). International Journal of Food and Fermentation Technology, 5, 231. https://doi.org/10.5958/2277-9396.2016.00006.4
Wu, S. C., & Lia, Y. K. (2008). Application of bacterial cellulose pellets in enzyme immobilization. J Mol Catal B Enzym, 54, 103–108. https://doi.org/10.1016/j.molcatb.2007.12.021.
Oontawee, S., Jittavisuttiwong, P., & Phonprapai, C. (2015). Physical properties of xyloglucan/bacterial cellulose composite film plasticized with glycerol. Key Engineering Materials, 659, 24–27. https://doi.org/10.4028/www.scientific.net/KEM.659.24
Cacicedo, M. L., Castro, M. C., Servetas, I., Bosnea, L., Boura, K., Tsafrakidou, P., Dima, A., Terpou, A., Koutinas, A., & Castro, G. R. (2016). Progress in bacterial cellulose matrices for biotechnological applications. Bioresource Technology, 213, 172–180. https://doi.org/10.1016/j.biortech.2016.02.071.
Wu, Z. Y., Liang, H. W., Chen, L. F., Hu, B. C., & Yu, S. H. (2016). Bacterial cellulose: A robust platform for design of three dimensional carbon-based functional nanomaterials. Accounts of Chemical Research, 49, 96–105. https://doi.org/10.1021/acs.accounts.5b00380
Jonas, R., & Farah, L. F. (1998). Production and application of microbial cellulose. Polymer Degradation and Stability, 59, 101–106. https://doi.org/10.1016/S0141-3910(97)00197-3.
Fu, L., Zhang, J., & Yang, G. (2013). Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydrate Polymers, 92, 1432–1442. https://doi.org/10.1016/j.carbpol.2012.10.071.
Rivas, B., Moldes, A. B., Domı́nguez, J. M., & Parajó, J. C. (2004). Development of culture media containing spent yeast cells of Debaryomyces hansenii and corn steep liquor for lactic acid production with Lactobacillus rhamnosus. International Journal of Food Microbiology, 97, 93–98. https://doi.org/10.1016/j.ijfoodmicro.2004.05.006.
Jozala, A. F., de Lencastre-Novaes, L. C., Lopes, A. M., de Carvalho Santos-Ebinuma, V., Mazzola, P. G., Pessoa-Jr, A., Grotto, D., Gerenutti, M., & Chaud, M. V. (2016). Bacterial nanocellulose production and application: A 10-year overview. Applied Microbiology and Biotechnology, 100, 2063–2072. https://doi.org/10.1007/s00253-015-7243-4.
Ruka, D. R., Simon, G. P., & Dean, K. M. (2012). Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydrate Polymers, 89, 613–622. https://doi.org/10.1016/j.carbpol.2012.03.059.
Lee, K. Y., Buldum, G., Mantalaris, A., & Bismarck, A. (2014). More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromolecular Bioscience, 14, 10–32. https://doi.org/10.1002/mabi.201300298
Aydın, Y. A., & Aksoy, N. D. (2014). Isolation and characterization of an efficient bacterial cellulose producer strain in agitated culture: Gluconacetobacter hansenii P2A. Applied Microbiology and Biotechnology, 98, 1065–1075. https://doi.org/10.1007/s00253-013-5296-9.
Lin, S. P., Liu, C. T., Hsu, K. D., Hung, Y. T., Shih, T. Y., & Cheng, K. C. (2016). Production of bacterial cellulose with various additives in a PCS rotating disk bioreactor and its material property analysis. Cellulose, 23, 367–377. https://doi.org/10.1007/s10570-015-0855-0.
Ramana, K. V., Tomar, A., & Singh, L. (2000). Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World Journal of Microbiology & Biotechnology, 16, 245–248. https://doi.org/10.1023/A:1008958014270
Oikawa, T., Morino, T., & Ameyama, M. (1995). Production of cellulose from D-arabitol by Acetobacter xylinum KU-1. Bioscience, Biotechnology, and Biochemistry, 59, 1564–1565. https://doi.org/10.1271/bbb.59.1564
Çoban, E. P., & Biyik, H. (2011). Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter lovaniensis HBB5. African Journal of Biotechnology, 10, 5346–5354. https://doi.org/10.5897/AJB10.1693.
Gea, S., Torres, F. G., Troncoso, O. P., Reynolds, C. T., Vilasecca, F., Iguchi, M., & Peijs, T. (2007). Biocomposites based on bacterial cellulose and Apple and Radish Pulp. International Polymer Processing, 22, 497–501. https://doi.org/10.3139/217.2059.
Espinoza Tapia, J. C., LeBorgne, S., Olivares Hernández, R., Hernández-Guerrero, M., González Reyes, L., & Vigueras-Ramírez, J. G. (2020). Validación de método analítico para la cuantificación de compuestos de fermentación por HPLC–RI–UV. Revista tediq, 6, 100–103.
Krystynowicz, A., Czaja, W., Wiktorowska-Jezierska, A., Gonçalves-Miśkiewicz, M., Turkiewicz, M., & Bielecki, S. (2002). Factors affecting the yield and properties of bacterial cellulose. Journal of Industrial Microbiology and Biotechnology, 29, 189–195. https://doi.org/10.1038/sj.jim.7000303
Pa'e, N., Zahan K. A., & Muhamad, I. I. (2011). Production of biopolymer from Acetobacter xylinum using different fermentation methods. International Journal of Engineering & Technology IJET-IJENS, 11, 90–97.
Jaramillo, R., Perna, O., Revollo, A. B., Arrieta, C., & Escamilla, E. (2013). Efecto de diferentes concentraciones de fructosa sobre la producción de celulosa bacteriana en cultivo estático. Revista Colombiana de Ciencia Animal - RECIA, 5, 116. https://doi.org/10.24188/recia.v5.n1.2013.476
Lindemann, S. R., Bernstein, H. C., Song, H. S., Fredrickson, J. K., Fields, M. W., Shou, W., Johnson, D. R., & Beliaev, A. S. (2016). Engineering microbial consortia for controllable outputs. Isme Journal, 10, 2077–2084. https://doi.org/10.1038/ismej.2016.26.
Jiang, L. L., Zhou, J. J., Quan, C. S., & Xiu, Z. L. (2017). Advances in industrial microbiome based on microbial consortium for biorefinery. Bioresour Bioprocess, 4, 11. https://doi.org/10.1186/s40643-017-0141-0.
Jayabalan, R., Malbaša, R. V., Lončar, E. S., Vitas, J. S., & Sathishkumar, M. (2014). A review on Kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Comprehensive Reviews in Food Science and Food Safety, 13, 538–550. https://doi.org/10.1111/1541-4337.12073
Vázquez-Cabral, B. D., Larrosa-Pérez, M., Gallegos-Infante, J. A., Moreno-Jiménez, M. R., González-Laredo, R. F., Rutiaga-Quiñones, J. G., Gamboa-Gómez, C. I., & Rocha-Guzmán, N. E. (2017). Oak Kombucha protects against oxidative stress and inflammatory processes. Chemico-Biological Interactions, 272, 1–9. https://doi.org/10.1016/j.cbi.2017.05.001
Chávez-Pacheco, J. L., Martínez-Yee, S., Contreras-Zentella, M., & Escamilla-Marván, E. (2004). Celulosa bacteriana en Gluconacetobacter xylinum: biosíntesis y aplicaciones. TIP Revista Especializada en Ciencias Químico-Biológicas, 7, 18–25.
Chakravorty, S., Bhattacharya, S., Chatzinotas, A., Chakraborty, W., Bhattacharya, D., & Gachhui, R. (2016). Kombucha tea fermentation: Microbial and biochemical dynamics. International Journal of Food Microbiology, 220, 63–72. https://doi.org/10.1016/j.ijfoodmicro.2015.12.015.
Marsh, A. J., O’Sullivan, O., Hill, C., Ross, R. P., & Cotter, P. D. (2014). Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiology, 38, 171–178. https://doi.org/10.1016/j.fm.2013.09.003.
Chawla, P. R., Bajaj, I. B., Survase, S. A. & Singhal, R. S. (2009). Microbial cellulose: Fermentative production and applications. Food Technology and Biotechnology, 47, 107–124.
Gea, S., Reynolds, C. T., Roohpour, N., Wirjosentono, B., Soykeabkaew, N., Bilotti, E., & Peijs, T. (2011). Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresource Technology, 102, 9105–9110. https://doi.org/10.1016/j.biortech.2011.04.077.
Tsalagkas, D., Lagaňa, R., Poljanšek, I., Oven, P., & Csoka, L. (2016). Fabrication of bacterial cellulose thin films self-assembled from sonochemically prepared nanofibrils and its characterization. Ultrasonics Sonochemistry, 28, 136–143. https://doi.org/10.1016/j.ultsonch.2015.07.010.
López-Simeon, R., Campos-Terán, J., Beltrán, H. I., & Hernández-Guerrero, M. (2012). Free-lignin cellulose obtained from agar industry residues using a continuous and minimal solvent reaction/extraction methodology. RSC Advances, 2, 12286. https://doi.org/10.1039/c2ra22185c
Simpson, L. P., & Riggs, C. (1983). Bleaching with sodium hypochlorite: Interactions of temperature, time, pH and concentration with stain removal and fabric strength. Journal of the American Oil Chemists Society, 60, 1680–1686. https://doi.org/10.1007/BF02662434.
Siró, I., & Plackett, D. (2010). Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose, 17, 459–494. https://doi.org/10.1007/s10570-010-9405-y.
Lee, Y. J., An, S. J., Bae, E. B., Gwon, H. J., Park, J. S., Jeong, S., Jeon, Y. C., Lee, S. H., Lim, Y. M., & Huh, J. B. (2017). The effect of thickness of resorbable bacterial cellulose membrane on guided bone regeneration. Materials, 10, 320. https://doi.org/10.3390/ma10030320
Tabaii, M. J., & Emtiazi, G. (2016). Comparison of bacterial cellulose production among different strains and fermented media. Applied Food Biotechnology, 3, 35–41. www.journals.sbmu.ac.ir/afb
Yano, H., Sugiyama, J., Nakagaito, A. N., Nogi, M., Matsuura, T., Hikita, M., & Handa, K. (2005). Optically transparent composites reinforced with networks of bacterial nanofibers. Advanced Materials, 17, 153–155. https://doi.org/10.1002/adma.200400597
Borzani, W., & de Souza, S. J. (1995). Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitaded liquid media. Biotechnology Letters, 17, 1271–1272. https://doi.org/10.1007/BF00128400.
Halib, N., Amin, M. C. I. M., & Ahmad, I. (2012). Physicochemical properties and characterization of nata de coco from local food industries as a source of cellulose. Sains Malaysiana, 41, 205–211.
Auta, R., Adamus, G., Kwiecien, M., Radecka, I., & Hooley, P. (2017). Production and characterization of bacterial cellulose before and after enzymatic hydrolysis. African Journal of Biotechnology, 16, 470–482. https://doi.org/10.5897/AJB2016.15486
Berggren, R., Berthold, F., Sjöholm, E., & Lindström, M. (2003). Improved methods for evaluating the molar mass distributions of cellulose in kraft pulp. Journal of Applied Polymer Science, 88, 1170–1179. https://doi.org/10.1002/app.11767
Feng, Y. H., Bin Wang, X., Lin, Q., Wu, Z. X., Pang, S. J., & Yin, X. Q. (2006). Biosynthesis of low molecular weight bacterial cellulose. Key Engineering Materials, 309–311, 497–502. https://doi.org/10.4028/www.scientific.net/KEM.309-311.497
Einfeldt, L., & Klemm, D. (1997). The control of cellulose biosynthesis by Acetobacter Xylinum in view of molecular weight and molecular weight distribution part I: Change of molecular weight of bacterial cellulose by simple variation of culture conditions. Journal of Carbohydrate Chemistry, 16, 635–646. https://doi.org/10.1080/07328309708007341.
Nainggolan, H., Gea, S., Bilotti, E., Peijs, T., & Hutagalung, S. D. (2013). Mechanical and thermal properties of bacterial-cellulose-fibre-reinforced Mater-Bi ® bionanocomposite. Beilstein Journal of Nanotechnology, 4, 325–329. https://doi.org/10.3762/bjnano.4.37.
Scheurle, A., Kunisch, E., Boccaccini, A. R., Walker, T., Renkawitz, T., & Westhauser, F. (2024). Boric acid and molybdenum trioxide synergistically stimulate osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Journal of Trace Elements in Medicine and Biology, 83, 127405. https://doi.org/10.1016/j.jtemb.2024.127405
Cortizo, M. C., & Lorenzo de Mele, M. F. (2004). Cytotoxicity of copper ions released from metal. Biological Trace Element Research, 102, 129–141.
Cao, B., Zheng, Y., Xi, T., Zhang, C., Song, W., Burugapalli, K., Yang, H., & Ma, Y. (2012). Concentration-dependent cytotoxicity of copper ions on mouse fibroblasts in vitro: Effects of copper ion release from TCu380A vs TCu220C intra-uterine devices. Biomedical Microdevices, 14, 709–720. https://doi.org/10.1007/s10544-012-9651-x.
Acknowledgements
The authors would like to acknowledge José David Sepúlveda Sánchez and Guadalupe Cruz-Barrera for assistance with SEM and GPC respectively, as well as LEACSA for providing access to the GPC extension software.
Funding
This work was supported by the Consejo Nacional de Humanidades, Ciencias y Tecnologías in Mexico, Projects B134267/47410235 and 287615, and the Programa de Mejoramiento del Profesorado (PROMEP-SEP), grants UAM-PTC-140 and No. 47410256.
Author information
Authors and Affiliations
Contributions
MHG: conceptualization, methodology, investigation, formal analysis, data curation, visualization, resources, supervision, funding acquisition, project administration, writing—original draft. DGM: visualization, writing—original draft. JGC: conceptualization, methodology, investigation, formal analysis. SR: resources, writing—review and editing. JCT: resources, writing—review and editing. GVR: conceptualization, methodology, investigation, formal analysis, visualization, resources, supervision, funding acquisition, project administration, writing—original draft
Corresponding author
Ethics declarations
Ethical Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
All authors have agreed to the publication of this work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández-Guerrero, M., Gomez-Maldonado, D., Gutiérrez-Castañeda, J. et al. Assessment of Culture Systems to Produce Bacterial Cellulose with a Kombucha Consortium. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04929-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04929-z