Abstract
In recent years, enzyme immobilization technology has been developed, and studies on immobilized enzyme materials have become very prominent. With the immobilization technique, enzymes and compatible carrier materials are combined or enzyme crystals/aggregates are used in a carrier-free fashion, by physical, chemical, or biochemical methods. As a kind of biocatalyst, immobilized enzymes can catalyze certain chemical reactions with high selectivity and high efficiency under relatively mild reaction conditions and eliminate pollution to the environment. Considering the current status and applications of immobilized enzyme technology and materials emerging in the last 5 years, this mini-review introduces the advantages and disadvantages of various enzyme immobilization techniques with carriers as well as the pros and cons of different materials for immobilization. The future prospects of immobilization technology and carrier materials are outlined, aiming to provide a reference for further research and applications of sustainable technology.
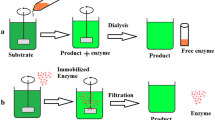
Graphical Abstract




Copyright 2021 American Chemical Society

Reproduced with permission from ref. [95]. Copyright by Spring Nature Publishing Group

Reproduced with permission from ref. [100]. Creative Commons CC BY license
Similar content being viewed by others
Data Availability
Not applicable. This is a review which does not contain research data.
References
Tosa, T., Mori, T., Fuse, N., & Chibata, F. (1969). Studies on continuous enzyme reactions, Part V. Kinetics and industrial applications of aminoacyl column for continuous optical resolution of acyl amino-acids. Agricultural and Biological Chemistry, 33, 1047–1052.
Tosa, T., Mori, T., Fuse, N., & Chibata, I. (1969). Studies on continuous enzyme reactions: part VI. Enzymatic properties of the DEAE-sephadex-aminoacylase complex. Agricultural and Biological Chemistry, 33, 1053–1059.
Rodrigues, R. C., Ortiz, C., Berenguer-Murcia, Á., Torres, R., & Fernández-Lafuente, R. (2013). Modifying enzyme activity and selectivity by immobilization. Chemical Society Reviews, 42(15), 6290–6307.
Zhang, H., Bai, Y., Zhu, N., & Xu, J. (2021). Microfluidic reactor with immobilized enzyme - from construction to applications: a review. Chinese Journal of Chemical Engineering, 30, 136–145.
Chen, K., Huang, X., Kan, S. B. J., Zhang, R. K., & Arnold, F. H. (2018). Enzymatic construction of highly strained carbocycles. Science, 360(6384), 71–75.
Cui, J., Ren, S., Lin, T., Feng, Y., & Jia, S. (2018). Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@silica core shell nanosphere. Chemical Engineering Journal, 343, 629–637.
Cui, J., Feng, Y., Lin, T., Tan, Z., Zhong, C., & Jia, S. (2017). Mesoporous metal–organic framework with well-defined cruciate flower-like morphology for enzyme immobilization. ACS Applied Materials & Interfaces, 9, 10587–10594.
Nestl, B. M., Hammer, S. C., Nebel, B. A., & Hauer, B. (2014). New generation of biocatalysts for organic synthesis. Angewandte Chemie International Edition, 53(12), 3070–3095.
Huo, M., Wang, L., Chen, Y., & Shi, J. (2017). Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nature Communications, 8, 357.
Zhou, J., Wu, Y., Zhang, Q., Xu, G., & Ni, Y. (2021). Co-immobilized alcohol dehydrogenase and glucose dehydrogenase with resin extraction for continuous production of chiral diaryl alcohol. Applied Biochemistry and Biotechnology, 193, 2742–2758.
Huo, M., Wang, L., Chen, Y., & Shi, J. (2020). Glucose-responsive cascaded nanocatalytic reactor with self-modulation of the tumor microenvironment for enhanced chemo-catalytic therapy. Materials Horizon, 7, 1834–1844.
Fu, C., Lu, T., Dai, X., Ding, P., Xiong, Y., Ge, J., & Li, X. (2023). Co-immobilization of enzymes and metals on the covalent-organic framework for the efficient removal of mycotoxins. ACS Applied Materials & Interfaces, 15, 6859–6867.
Braham, S. A., Morellon-Sterling, R., de Andrades, D., Rodrigues, R. C., Siar, E.-H., Aksas, A., Pedroche, J., Millán, M. C., & Fernandez-Lafuente, R. (2021). Effect of tris buffer in the intensity of the multipoint covalent immobilization of enzymes in glyoxyl-agarose beads. Applied Biochemistry and Biotechnology, 193, 2843–2857.
Li, T., Qiu, H., Liu, N., Li, J., Bao, Y., & Tong, W. (2020). Construction of self-activated cascade metal-organic framework/enzyme hybrid nanoreactors as antibacterial agents. Colloids and Surfaces B: Biointerfaces, 191, 111001.
Sasaki, K., Furusawa, H., Nagamine, K., & Tokito, S. (2020). Constructive optimization of a multienzymatic film based on a cascade reaction for electrochemical biosensors. ACS Omega, 5(50), 32844–32851.
Sun, K., Ding, Z., Zhang, J., Chen, H., Qin, Y., Xu, S., Wu, C., Yu, J., & Chiu, D. T. (2021). Enhancing the long-term stability of a polymer dot glucose transducer by using an enzymatic cascade reaction system. Advanced Healthcare Materials, 10(4), 2001019.
Wang, T., Lei, Q.-L., Wang, M., Deng, G., Yang, L., Liu, X., Li, C., Wang, Q., Liu, Z., Wang, J., Cui, Z., Utama, K. G., Ni, R., & Chen, X. (2020). Mechanical tolerance of cascade bioreactions via adaptive curvature engineering for epidermal bioelectronics. Advanced Materials, 32(22), 2000991.
Bolivar, J. M., Woodley, J. M., & Fernandez-Lafuente, R. (2022). Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chemical Society Reviews, 51, 6251–6290.
Maghraby, Y. R., El-Shabasy, R. M., Ibrahim, A. H., & Azzazy, H. M. E. (2023). Enzyme immobilization technologies and industrial applications. ACS Omega, 8, 5184–5196.
Wunschik, D. S., Lorenz, A., Ingenbosch, K. N., Gutmann, J. S., & Hoffmann-Jacobsen, K. (2022). Activation and stabilization of lipase B from Candida antarctica by immobilization on polymer brushes with optimized surface structure. Applied Biochemistry and Biotechnology, 194, 3384–3399.
Mohammadi, M., Shahedi, M., Ahrari, F., Mostafavi, M., Habibi, Z., & Yousefi, M. (2023). Isocyanide-based multi-component reactions for carrier-free and carrier-bound covalent immobilization of enzymes. Nature Protocols, 18, 1641–1657.
López-Gallego, F., Fernandez-Lorente, G., Rocha-Martín, J., Bolivar, J. M., Mateo, C., & Guisan, J. M. (2020). Multi-point covalent immobilization of enzymes on glyoxyl agarose with minimal physicochemical modification: stabilization of industrial enzymes. Methods in Molecular Biology, 2100, 93–107.
Kermasha, S., & Gill, J. K. (2021) Chapter 6 - Immobilization of enzymes and their use in biotechnological applications. in ENZYMES, Novel Biotechnological Approaches for the Food Industry. S. Kermasha and M. N. A. Eskin. Academic Press, pp. 133–170.
Yuan, Y., Shen, J., & Salmon, S. (2023). Developing enzyme immobilization with fibrous membranes: longevity and characterization considerations. Membranes, 13(5), 532.
Fu, J., Reinhold, J., & Woodbury, N. W. (2011). Peptide-modified surfaces for enzyme immobilization. PLoS One, 6(4), e18692.
Mateo, C., Palomo, J. M., Fuentes, M., Betancor, L., Grazu, V., López-Gallego, F., Pessela, B. C. C., Hidalgo, A., Fernández-Lorente, G., Fernández-Lafuente, R., & Guisán, J. M. (2006). Glyoxyl agarose: A fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme and Microbial Technology, 39(2), 274–280.
Lee, D., Park, C., Yeo, J., & Kim, S. (2006). Lipase immobilization on silica gel using a cross-linking method. Journal of Industrial and Engineering Chemistry, 12(5), 777–782.
Imam, H. T., Marr, P. C., & Mar, A. C. (2021). Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chemistry, 23, 4980–5005.
de Andrade Silva, T., Keijok, W. J., Guimarães, M. C. C., Cassini, S. T. A., & de Oliveira, J. P. (2022). Impact of immobilization strategies on the activity and recyclability of lipases in nanomagnetic supports. Scientific Reports, 12(1), 6815.
Shivudu, G., Chandraraj, K., & Selvam, P. (2020). Production of xylooligosaccharides from xylan catalyzed by endo-1,4-β-D-xylanase-immobilized nanoscale carbon, silica and zirconia matrices. Molecular Catalysis, 484, 110745.
Chen, N., Zhang, C., Liu, Y., Dong, X., & Sun, Y. (2019). Cysteine-modified poly (glycidyl methacrylate) grafted onto silica nanoparticles: new supports for significantly enhanced performance of immobilized lipase. Biochemical Engineering Journal, 145, 137–144.
Pietricola, G., Ottone, C., Fino, D., & Tommasi, T. (2020). Enzymatic reduction of CO2 to formic acid using FDH immobilized on natural zeolite. Journal of CO2 Utilization, 42, 101343.
Zou, B., Chu, Y., Xia, J., Chen, X., & Huo, S. (2018). Immobilization of lipase by ionic liquid-modified mesoporous SiO2 adsorption and calcium alginate-embedding method. Applied Biochemistry and Biotechnology, 2018(185), 606–618.
Imam, H. T., Hill, K., Reid, A., Mix, S., Marr, P. C., & Marr, A. C. (2023). Supramolecular ionic liquid gels for enzyme entrapment. ACS Sustainable Chemistry & Engineering, 11(18), 6829–6837.
Chen, B., Wang, X., Gao, X., Jiang, J., Hu, M., Li, S., & Jiang, Y. (2021). DNA directed immobilization of horseradish peroxidase on phase-transitioned lysozyme modified TiO2 for efficient degradation of phenol in wastewater. Materials & Design, 201, 109463.
Chen, J., Sun, B., Sun, C., Zhang, P., Xu, W., Liu, Y., & Tang, K. (2020). Immobilization of lipase AYS on UiO-66-NH2 metal-organic framework nanoparticles as a recyclable biocatalyst for ester hydrolysis and kinetic resolution. Separation and Purification Technology, 251, 117398.
Chauhan, V., Kaushal, D., Dhiman, V. K., Kanwar, S. S., Singh, D., Dhiman, V. K., & Pandey, H. (2022). An insight in developing carrier-free immobilized enzymes. Frontiers in Bioengineering and Biotechnology, 10, 794411.
Chen, N., Chang, B., Shi, N., Yan, W., Lu, F., & Liu, F. (2023). Cross-linked enzyme aggregates immobilization: preparation, characterization, and applications. Critical Reviews in Biotechnology, 43(3), 369–383.
Öndeş, B., Uygun, M., Evli, S., & Uygun, D. A. (2022). Immobilization of urokinase onto magnetically directed micromotors. Applied Biochemistry and Biotechnology, 194, 3351–3364.
Smeets, V., Baaziz, W., Ersen, O., Gaigneaux, E. M., Boissière, C., Sanchez, C., & Debecker, D. P. (2019). Hollow zeolite microspheres as a nest for enzymes: a new route to hybrid heterogeneous catalysts. Chemical Science, 11, 954–961.
Van der Verren, M., Smeets, V., Straeten, A. V., Dupont-Gillain, C., & Debecker, D. P. (2021). Hybrid chemoenzymatic heterogeneous catalyst prepared in one step from zeolite nanocrystals and enzyme-polyelectrolyte complexes. Nanoscale Advances, 3(6), 1646–1655.
Doğan, D., Sezer, S., Ulu, A., Köytepe, S., & Ateş, B. (2021). Preparation and characterization of amino-functionalized zeolite/SiO2 materials for trypsin–chymotrypsin co-immobilization. Catalysis Letters, 151, 2463–2477.
Qayoudi, A. A., & Al-Zuhair, S. (2022). Dynamic modelling of enzymatic hydrolysis of oil using lipase immobilized on zeolite. Sustainability, 14(14), 8399.
Lee, S. Y., Show, P. L., Ko, C.-M., & Chang, Y.-K. (2019). A simple method for cell disruption by immobilization of lysozyme on the extrudate-shaped Na-Y zeolite: recirculating packed bed disruption process. Biochemical Engineering Journal, 141, 210–216.
Kujawa, J., Głodek, M., Koter, I., Li, G., Knozowska, K., & Kujawski, W. (2022). Bioconjugation strategy for ceramic membranes decorated with Candida antarctica lipase B—impact of immobilization process on material features. Materials, 15(2), 671.
Mulinari, J., Oliveira, J. V., & Hotza, D. (2020). Lipase immobilization on ceramic supports: an overview on techniques and materials. Biotechnology Advances, 42, 107581.
Valotta, A., Maier, M. C., Soritz, S., Pauritsch, M., Koenig, M., Brouczek, D., Schwentenwein, M., & Gruber-Woelfler, H. (2021). 3D printed ceramics as solid supports for enzyme immobilization: an automated DoE approach for applications in continuous flow. Journal of Flow Chemistry, 11(3), 675–689.
Vakili, F., Mojtabavi, S., Imanparast, S., Kianmehr, Z., Forootanfar, H., & Faramarzi, M. A. (2020). Immobilization of lipase on the modified magnetic diatomite earth for effective methyl esterification of isoamyl alcohol to synthesize banana flavor. 3 Biotech, 10, 447.
M. P. Cabrera, T. França da Fonseca, R. V. B. de Souza, C. R. D. de Assis, J. Q. Marcatoma, J. da C. Maciel, D. F. M. Neri, F. Soria, & L. B. de Carvalho Jr. (2018). Polyaniline-coated magnetic diatomite nanoparticles as a matrix for immobilizing enzymes. Applied Surface Science 457, 21-29.
Guo, J., Liu, X., Zhang, X., Wu, J., Chai, C., Ma, D., Chen, Q., Xiang, D., & Ge, W. (2019). Immobilized lignin peroxidase on Fe3O4@SiO2@polydopamine nanoparticles for degradation of organic pollutants. International Journal of Biological Macromolecules, 138, 433–440.
Li, L., Zhang, W., Wei, Y., Yu, L., & Feng, D. (2022). A sensitive fluorescent immunoassay for prostate specific antigen detection based on signal amplify strategy of horseradish peroxidase and silicon dioxide nanospheres. Journal of Analytical Methods in Chemistry, 2022, 6209731.
Shen, Y., Zhang, Y., Zhang, X., Zhou, X., Teng, X., Yan, M., & Bi, H. (2015). Horseradish peroxidase-immobilized magnetic mesoporous silica nanoparticles as a potential candidate to eliminate intracellular reactive oxygen species. Nanoscale, 7(7), 2941–2950.
Luo, Y., Jin, D., He, W., Huang, J., Chen, A., & Qi, F. (2021). A SiO2 microcarrier with an opal-like structure for cross-linked enzyme immobilization. Langmuir, 37, 14147–14156.
Ahmed, S. A., Mostafa, F. A., & Ouis, M. A. (2018). Enhancement stability and catalytic activity of immobilized α-amylase using bioactive phospho-silicate glass as a novel inorganic support. International Journal of Biological Macromolecules, 112, 371–382.
Hosseini, S. S., Khodaiyan, F., Mousavi, E. S. M., Azimi, S. Z., & Gharaghani, M. (2020). Immobilization of pectinase on the glass bead using polyaldehyde kefiran as a new safe cross-linker and its effect on the activity and kinetic parameters. Food Chemistry, 309, 125777.
Wang, D., Hartz, W. F., & Moloney, M. G. (2023). Surface modified materials for active capture of enzymes. Journal of Materials Chemistry B, 11, 2377–2388.
Pounsamy, M., Somasundaram, S., Palanivel, S., Balasubramani, R., Chang, S. W., Nguyen, D. D., & Ganesan, S. (2019). A novel protease-immobilized carbon catalyst for the effective fragmentation of proteins in high-TDS wastewater generated in tanneries: Spectral and electrochemical studies. Environmental Research, 172, 408–419.
Santos, M. P. F., Porfírio, M. C. P., Junior, E. C. S., Bonomo, R. C. F., & Veloso, C. M. (2022). Pepsin immobilization: influence of carbon support functionalization. International Journal of Biological Macromolecules, 203, 67–79.
Lee, A. A., Gervasio, E. D., Hughes, R. O., Maalouf, A. A., Musso, S. A., Crisalli, A. M., & Woolridge, E. M. (2023). Alginate encapsulation stabilizes xylanase toward the laccase mediator system. Applied Biochemistry and Biotechnology, 195, 3311–3326.
Maity, M., Bhattacharyya, A., & Bhowal, J. (2021). Production and immobilization of β-galactosidase isolated from Enterobacter aerogenes KCTC2190 by entrapment method using agar-agar organic matrix. Applied Biochemistry and Biotechnology, 193, 2198–2224.
Zhou, Y., Dong, J., & Wang, Q. (2023). Fabricating higher-order functional DNA origami structures to reveal biological processes at multiple Scales. NPG Asia Materials, 15, 25.
Zhan, P., Peil, A., Jiang, Q., Wang, D., Mousavi, S., Xiong, Q., Shen, Q., Shang, Y., Ding, B., Lin, C., Ke, Y., & Liu, N. (2023). Recent Advances in DNA origami-engineered nanomaterials and applications. Chemical Reviews, 123(7), 3976–4050.
Kahn, J. S., Xiong, Y., Huang, J., & Gang, O. (2022). Cascaded enzyme reactions over a three-dimensional, wireframe DNA origami scaffold. JACS Au, 2, 357–366.
Lin, P., Dinh, H., Morita, Y., Zhang, Z., Nakata, E., Kinoshita, M., & Morii, T. (2021). Evaluation of the role of the DNA surface for enhancing the activity of scaffolded enzymes. Chemical Communications, 57(32), 3925–3928.
Dwamena, A. K., Woo, S. H., & Kim, C. S. (2020). Enzyme immobilization on porous chitosan hydrogel capsules formed by anionic surfactant gelation. Biotechnology Letters, 42(5), 845–852.
Costa, G. P., Spolidoro, L. S., Manfroi, V., Rodrigues, R. C., & Hertz, P. F. (2022). α-Acetolactate decarboxylase immobilized in chitosan: A highly stable biocatalyst to prevent off-flavor in beer. Biotechnology Progress, 38(6), e3295.
Gür, S. D., İdil, N., & Aksöz, N. (2018). Optimization of enzyme co-immobilization with sodium alginate and glutaraldehyde-activated chitosan beads. Applied Biochemistry and Biotechnology, 184(2), 538–552.
Zhang, H., Nie, M., Gu, Z., Xin, Y., Zhang, L., Li, Y., & Shi, G. (2023). Preparation of water-insoluble lignin nanoparticles by deep eutectic solvent and its application as a versatile and biocompatible support for the immobilization of α-amylase. International Journal of Biological Macromolecules, 249, 125975.
Verma, N. K., & Raghav, N. (2022). Cellulose tosylate as support for α-amylase immobilization. International Journal of Biological Macromolecules, 222, 413–420.
Abdel Wahab, W. A., Karam, E. A., Hassan, M. E., Kansoh, A. L., Esawy, M. A., & Awad, G. E. A. (2018). Optimization of pectinase immobilization on grafted alginate-agar gel beads by 24 full factorial CCD and thermodynamic profiling for evaluating of operational covalent immobilization. International Journal of Biological Macromolecules, 113, 159–170.
Zhou, L., Liu, Y., Shi, H., Yang, X., Huang, J., Liu, S., & Wang, K. (2018). Flexible assembly of an enzyme cascade on a DNA triangle prism nanostructure for the controlled biomimetic generation of nitric oxide. ChemBioChem, 19(19), 2099–2106.
Wang, Z., St. Iago-Mcrae, E., Ebrahimimojarad, A., Won Oh, S., & Fu, J. (2022). Modulation of enzyme cascade activity by local substrate enrichment and exclusion on DNA nanostructures. Langmuir, 38(41),12594-12601.
Mela, I., Vallejo-Ramirez, P. P., Makarchuk, S., Christie, G., Bailey, D., Henderson, R. M., & Kaminski, C. F. (2020). DNA nanostructures for targeted antimicrobial delivery. Angewandte Chemie International Edition, 132(31), 12798–12802.
Lin, P., Dinh, H., & Morita, Y. (2023). Dynamic assembly of cascade enzymes by the shape transformation of a DNA scaffold. Advanced Functional Materials, 33(15), 2215023.
Kumari, A., & Kayastha, A. M. (2011). Immobilization of soybean (glycine max) α-amylase onto chitosan and amberlite MB-150 beads: Optimization and characterization. Journal of Molecular Catalysis B: Enzymatic, 69(1–2), 8–14.
Ashraf, H., & Husain, Q. (2010). Use of DEAE cellulose adsorbed and crosslinked white radish (Raphanus sativus) peroxidase for the removal of α-naphthol in batch and continuous process. International Biodeterioration & Biodegradation, 64(1), 27–31.
Hakkoymaz, O., & Mazi, H. (2020). An immobilized invertase enzyme for the selective determination of sucrose in fruit juices. Analytical Biochemistry, 611, 114000.
Shen, J., Qiao, J., Zhang, X., & Qi, L. (2021). Dual-stimuli-responsive porous polymer enzyme reactor for tuning enzymolysis efficiency. Microchimica Acta, 188(12), 435.
Kübelbeck, S., Mikhael, J., Keller, H., Konradi, R., Andrieu-Brunsen, A., & Baier, G. (2018). Enzyme–polymer conjugates to enhance enzyme shelf life in a liquid detergent formulation. Macromolecular Bioscience, 18(7), 1800095.
Rainer, T., Egger, A. S., Zeindl, R., Tollinger, M., Kwiatkowski, M., & Müller, T. (2022). 3D-printed high-pressure-resistant immobilized enzyme microreactor (ΜIMER) for protein analysis. Analytical Chemistry, 94(24), 8580–8587.
Plekhanova, Y. V., Tikhonenko, S. A., Dubrovsky, A. V., Kim, A. L., Musin, E. V., Wang, G. J., & Reshetilov, A. N. (2019). Comparative study of electrochemical sensors based on enzyme immobilized into polyelectrolyte microcapsules and into chitosan gel. Analytical Sciences, 35(9), 1037–1043.
Liao, Y., Wang, X., Shen, H., Tai, Z., & Wang, Q. (2022). Dynamic assembly and biocatalysis-selected gelation endow self-compartmentalized multienzyme superactivity. Science China Chemistry, 65, 1985–1993.
Pinyakit, Y., Romphophak, P., Painmanakul, P., & Hoven, V. P. (2023). Introduction of an ambient 3D-printable hydrogel ink to fabricate an enzyme-immobilized platform with tunable geometry for heterogeneous biocatalysis. Biomacromolecules, 24(7), 3138–3148.
Wang, D., Cui, F., Xi, L., Tan, X., Li, J., & Li, T. (2023). Preparation of a multifunctional non-stick tamarind polysaccharide-polyvinyl alcohol hydrogel immobilized with a quorum quenching enzyme for maintaining fish freshness. Carbohydrate Polymers, 302, 120382.
Bilal, M., Rasheed, T., Zhao, Y., & Iqbal, H. M. (2019). Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. International Journal of Biological Macromolecules, 124, 742–749.
Lin, H., Li, M., Ding, L., & Huang, J. (2019). A fiber optic biosensor based on hydrogel-immobilized enzyme complex for continuous determination of cholesterol and glucose. Applied Biochemistry and Biotechnology, 187, 1569–1580.
Qin, Z., Feng, N., Li, Y., Fei, X., Tian, J., Xu, L., & Wang, Y. (2022). Hydrogen-bonded lipase-hydrogel microspheres for esterification application. Journal of Colloid and Interface Science, 606, 1229–1238.
Zarei, A., Alihosseini, F., Parida, D., Nazir, R., & Gaan, S. (2021). Fabrication of cellulase catalysts immobilized on a nanoscale hybrid polyaniline/cationic hydrogel support for the highly efficient catalytic conversion of cellulose. ACS Applied Materials & Interfaces, 13(42), 49816–49827.
Chen, Q., Wang, Y., & Luo, G. (2023). Recycling of cofactors in crude enzyme hydrogels as co-immobilized heterogeneous biocatalysts for continuous-flow asymmetric reduction of ketones. Chemsuschem, 16(3), e202201654.
Liang, W., Wied, P., Carraro, F., Sumby, C. J., Nidetzky, B., Tsung, C.-K., Falcaro, P., & Doonan, C. J. (2021). Metal-organic framework-based enzyme biocomposites. Chemical Reviews, 121(3), 1077–1129.
Hu, C., Bai, Y., Hou, M., Wang, Y., Wang, L., Cao, X., Chan, C.-W., Sun, H., Li, W., Ge, J., & Ren, K. (2020). Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Science Advances, 6, eaax5785.
Ren, S., Wang, F., Gao, H., Han, X., Zhang, T., Yuan, Y., & Zhou, Z. (2023). Recent progress and future prospects of laccase immobilization on MOF supports for industrial applications. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-023-04607-6
Cheng, Q., Chi, X., Liang, Y., Li, W., Sun, J., Tao, J., & Wang, Z. (2023). Immobilization of lipase in Cu-BTC MOF with enhanced catalytic performance for resolution of N-hydroxymethyl vince lactam. Applied Biochemistry and Biotechnology, 195, 1216–1230.
He, W., Shen, H., Zhou, Z., Huang, Z., Chao, H., Song, J., Su, P., & Yang, Y. (2021). Janus DNA bridges metal-organic frameworks and graphene oxide for convenient and efficient multienzyme co-immobilization with boosted activity. Applied Surface Science, 570, 151242.
Chen, W.-H., Vázquez-González, M., Zoabi, A., Abu-Reziq, R., & Willner, I. (2018). Biocatalytic cascades driven by enzymes encapsulated in metal–organic framework nanoparticles. Nature Catalysis, 1, 689–695.
Zhou, X., Guo, S., Gao, J., Zhao, J., Xue, S., & Xu, W. (2017). Glucose oxidase-initiated cascade catalysis for sensitive impedimetric aptasensor based on metal-organic frameworks functionalized with Pt nanoparticles and hemin/G-quadruplex as mimicking peroxidases. Biosensors & Bioelectronics, 98, 83–90.
Zhang, Y., Xu, L., & Ge, J. (2022). Multienzyme system in amorphous metal-organic frameworks for intracellular lactate detection. Nano Letters, 22, 5029–5036.
Kandambeth, S., Venkatesh, V., Shinde, D. B., Kumari, S., Halder, A., Verma, S., & Banerjee, R. (2015). Self-templated chemically stable hollow spherical covalent organic framework. Nature Communications, 6, 6786.
Wang, B., Lin, R.-B., Zhang, Z., Xiang, S., & Chen, B. (2020). Hydrogen-bonded organic frameworks as a tunable platform for functional materials. Journal of the American Chemical Society, 142(34), 14399–14416.
Chen, G., Tong, L., Huang, S., Huang, S., Zhu, F., & Ouyang, G. (2022). Hydrogen-bonded organic framework biomimetic entrapment allowing non-native biocatalytic activity in enzyme. Nature Communications, 13(1), 4816.
Chen, J., Sun, B., Sun, C., Zhang, P., Xu, W., Liu, Y., Xiong, B., & Tang, K. (2020). Immobilization of lipase AYS on UiO-66-NH2 metal-organic framework nanoparticles as a recyclable biocatalyst for ester hydrolysis and kinetic resolution. Separation and Purification Technology, 251, 117398.
Elmerhi, N., Al-Maqdi, K., Athamneh, K., Mohammed, A. K., Skorjanc, T., Gándara, F., Raya, J., Pascal, S., Siri, O., Trabolsi, A., Shah, I., Shetty, D., & Ashraf, S. S. (2023). Enzyme-immobilized hierarchically porous covalent organic framework biocomposite for catalytic degradation of broad-range emerging pollutants in water. Journal of Hazardous Materials, 459, 132261.
Li, M., Qiao, S., Zheng, Y., Andaloussi, Y. H., Li, X., Zhang, Z., Li, A., Cheng, P., Ma, S., & Chen, Y. (2020). Fabricating covalent organic framework capsules with commodious microenvironment for enzymes. Journal of the American Chemical Society, 142(14), 6675–6681.
Zheng, Y., Zhang, S., Guo, J., Shi, R., Yu, J., Li, K., Li, N., Zhang, Z., & Chen, Y. (2022). Green and scalable fabrication of high-performance biocatalysts using covalent organic frameworks as enzyme carriers. Angewandte Chemie International Edition, 61, e202208744.
Chen, G., Huang, S., Ma, X., He, R., & Ouyang, G. (2023). Encapsulating and stabilizing enzymes using hydrogen-bonded organic frameworks. Nature Protocols, 18(7), 2032–2050.
Zhang, Y., Xing, C., Mu, Z., Niu, Z., Feng, X., Zhang, Y., & Wang, B. (2023). Harnessing self-repairing and crystallization processes for effective enzyme encapsulation in covalent organic frameworks. Journal of the American Chemical Society, 145(24), 13469–13475.
Liu, S., & Sun, Y. (2023). Co-encapsulating cofactor and enzymes in hydrogen-bonded organic frameworks for multienzyme cascade reactions with cofactor recycling. Angewandte Chemie International Edition, 62, e202308562.
Liang, J., Ruan, J., Njegic, B., Rawal, A., Jason, S., Xu, J., Boyer, C., & Liang, K. (2023). Insight into bioactivity of in-situ trapped enzyme-covalent-organic frameworks. Angewandte Chemie International Edition, 62, e202303001.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 21973061) and Zhejiang Provincial Science and Technology Innovation Program for College Students (Grant number 2023R465014).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by YJ, JZ, MW, WX, and YW. YJ, JZ, and LW performed the literature search and revised and reviewed the manuscript. JD performed the literature search, data analysis, revised and reviewed the manuscript, and supervised the study. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This is a review study which does not involve human or animal subjects and does not require ethics approval.
Consent to Participate
Not applicable.
Consent for Publication
This is a review study. The authors affirm that provided informed consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Zheng, J., Wang, M. et al. Pros and Cons in Various Immobilization Techniques and Carriers for Enzymes. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-023-04838-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04838-7