Abstract
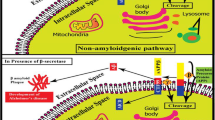
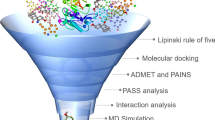
Alzheimer’s disease (AD) is an extremely complex, heterogeneous, and multifactorial neurodegenerative disease clinically characterized by progressive memory loss and progressive decline in cognitive function. There is currently no effective treatment for the onset and/or progression of the pathophysiological diseases of AD. The global prevalence of this disease has increased in recent years due to modern lifestyle. Therefore, there is an urgent need to develop a drug with significant neuroprotective potential. Since plant metabolites, especially polyphenols, have important pharmacological properties acting against β-amyloid (Aβ), Tau, neuroinflammation, and oxidative stress, such phytochemicals were selected in the present research. Using the Schrödinger tool (Maestro V.13.6), the drug potency of these metabolites was studied after installation in the highly configured workstation. Among the 120 polyphenols docked, amygdalin showed notable docking values of − 11.2638, followed by eriocitrin (− 10.9569), keracyanin (− 10.7086), and amaroswerin (− 9.48126). The prominent MM-GBSA values of these molecules were − 62.8829, − 52.1914, − 68.6307, and − 63.1074, respectively. The MM-GBSA energy values demonstrated the drug stability of these molecules for β-site amyloid precursor protein-cleaving enzyme 1 (BACE1)-causing AD. In the absorption and distribution assessment, these phytochemicals showed significantly better values than the inhibitors CNP520. The chosen phytochemicals have been demonstrated as non-hepatotoxic; however, the BACE1 inhibitor CNP520 is hepatotoxic. In both the molecular docking and ADMET assessments, these natural chemicals have shown optimism as potential drug candidates for Alzheimer’s disease. However, in order to understand the detailed biological metabolism of these compounds in AD, they need to be evaluated in in vivo studies to validate its efficacy.














Similar content being viewed by others
Data Availability
Data will be shared based on the request.
References
ADI. (2022). Alzheimer’s disease international report. https://www.alzint.org/about/. Accessed on 2.07.2023
Masters, C. L., Bateman, R., Blennow, K., Rowe, C. C., Sperling, R. A., & Cummings, J. L. (2015). Alzheimer’s disease. Nature Reviews Drug Discovery Primers, 1, 1–18. https://doi.org/10.1038/nrdp.2015.56
Golde, T. E., Dickson, D., & Hutton, M. (2006). Filling the gaps in the abeta cascade hypothesis of Alzheimer’s disease. Current Alzheimer Research, 3(5), 421–430. https://doi.org/10.2174/156720506779025189
Cole, S. L., & Vassar, R. (2007). The Alzheimer’s disease beta-secretase enzyme, BACE1. Molecular Neurodegeneration, 15(2), 22. https://doi.org/10.1186/1750-1326-2-22
De Strooper, B., Iwatsubo, T., & Wolfe, M. S. (2012). Presenilins and γ-secretase: Structure, function, and role in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 2, a006304. https://doi.org/10.1101/cshperspect.a006304
Janelidze, S., Zetterberg, H., Mattsson, N., Palmqvist, S., Vanderstichele, H., Lindberg, O., Westen, D., Stomrud, E., Minthon, L., Blennow, K., & Hansson, O. (2016). CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: Better diagnostic markers of Alzheimer disease. Annals of Clinical Translational Neurology, 3(3), 154–165. https://doi.org/10.1002/acn3.274
Koelsch, G. (2017). BACE1 Function and inhibition: Implications of intervention in the amyloid pathway of Alzheimer’s disease pathology. Molecules, 22(10), 1723. https://doi.org/10.3390/molecules22101723
Mullard, A. (2017). BACE inhibitor bust in Alzheimer trial. Nature Reviews Drug Discovery, 16, 155.
Wightman, E. (2017). Potential benefits of phytochemicals against Alzheimer’s disease. The Proceedings of the Nutrition Society, 76(2), 106–112. https://doi.org/10.1017/S0029665116002962
Coimbra, J. R. M., Marques, D. F. F., Baptista, S. J., Pereira, C. M. F., Moreira, P. I., Dinis, T. C. P., Santos, A. E., & Salvador, J. A. R. (2018). Highlights in BACE1 inhibitors for Alzheimer’s disease treatment. Frontiers in Chemistry, 24(6), 178. https://doi.org/10.3389/fchem.2018.00178
Sajad, M., Kumar, R., & Thakur, S. C. (2022). History in perspective: The prime pathological players and role of phytochemicals in Alzheimer’s disease. IBRO Neuroscience Reports, 28(12), 377–389. https://doi.org/10.1016/j.ibneur.2022.04.009
Rani, M. J., & J., Kalaimathi, K., Vijayakumar, S., Varatharaju, G., Karthikeyan, K., Thiyagarajan, G., Bhavani, K., Manogar, P., & Prabhu, S. (2022). Anti-viral effectuality of plant polyphenols against mutated dengue protein NS2B47-NS3: A computational exploration. Gene Reports, 27. https://doi.org/10.1016/j.genrep.2022.101546
Wang, X., Kim, J. R., Lee, S. B., Kim, Y. J., Jung, M. Y., Kwon, H. W., & Ahn, Y. J. (2014). Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer’s disease drosophila models.
Cox, C. J., Choudhry, F., Peacey, E., Perkinton, M. S., Richardson, J. C., Howlett, D. R., Lichtenthaler, S. F., Francis, P. T., & Williams, R. J. (2015). Dietary (-)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing. Neurobiology of Aging, 36, 178–187. https://doi.org/10.1016/j.neurobiolaging.2014.07.032
Guo, Y., Zhao, Y., Nan, Y., Wang, X., Chen, Y., & Wang, S. (2017). (-)-Epigallocatechin-3-gallate ameliorates memory impairment and rescues the abnormal synaptic protein levels in the frontal cortex and hippocampus in a mouse model of Alzheimer’s disease. NeuroReport, 28, 590–597. https://doi.org/10.1097/WNR.0000000000000803
Gaamouch, F., Chen, F., Ho, L., Lin, H. Y., Yuan, C., Wong, J., & Wang, J. (2022). Benefits of dietary polyphenols in Alzheimer’s disease. Frontiers in Aging Neuroscience, 13(14), 1019942. https://doi.org/10.3389/fnagi.2022
Moertel, C. G., Fleming, T. R., Rubin, J., Kvols, L. K., Sarna, G., Koch, R., Currie, V. E., Young, C. W., Jones, S. E., & Davignon, J. P. (1982). A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. New England Journal of Medicine, 306(4), 201–206. https://doi.org/10.1056/NEJM198201283060403
Swain, E., & Poulton, J. E. (1994). Utilization of amygdalin during seedling development of Prunus serotina. Plant Physiology, 106(2), 437–445. https://doi.org/10.1104/pp.106.2.437
Bolarinwa, I. F., Orfila, C., & Morgan, M. R. (2014). Amygdalin content of seeds, kernels and food products commercially available in the UK. Food Chemistry, 152, 133–139. https://doi.org/10.1016/j.foodchem.2013.11.002
Barceloux, D. G. (2009). Cyanogenic foods (cassava, fruit kernels, and cycad seeds). Disease-a-Month, 55(6), 336–352. https://doi.org/10.1016/j.disamonth.2009.03.010
Yang, H. Y., Chang, H. K., Lee, J. W., Kim, Y. S., Kim, H., Lee, M. H., Shin, M. S., Ham, D. H., Park, H. K., Lee, H., & Kim, C. J. (2007). Amygdalin suppresses lipopolysaccharide-induced expressions of cyclooxygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Neurological Research, 29(1), 59–S64. https://doi.org/10.1179/016164107X172248
Jiagang, D., Li, C., Wang, H., Hao, E., Du, Z., Bao, C., Lv, J., & Wang, Y. (2011). Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice through the induction of regulatory T cells. Biochemical and Biophysical Research Communications, 411(3), 523–529. https://doi.org/10.1016/j.bbrc.2011.06.162
Taiwo, I. A., Odeigah, P. G. C., Jaja, S., & Mojiminiyi, F. (2010). Cardiovascular effects of Vernonia amygdalina in rats and the implications for treatment of hypertension in diabetes. Researcher, 2, 76–79.
Yang, C., Zhao, J., Cheng, Y., Li, X., & Rong, J. (2014). Bioactivity-guided fractionation identifies amygdalin as a potent neurotrophic agent from herbal medicine Semen Persicae extract. Biomed Research International, 306857. https://doi.org/10.1155/2014/306857
Vahedi-Mazdabadi, Y., Karimpour-Razkenari, E., Akbarzadeh, T., Lotfian, H., Toushih, M., Roshanravan, N., Saeedi, M., & Ostadrahimi, A. (2020). Anti-cholinesterase and neuroprotective activities of sweet and bitter apricot kernels (Prunus armeniaca L). Iranian Journal Pharmaceutical Research: IJPR, 19(4), 216. https://doi.org/10.22037/2Fijpr.2019.15514.13139
Moslehi, A., Komeili-Movahed, T., & Moslehi, M. (2019). Antioxidant effects of amygdalin on tunicamycin-induced endoplasmic reticulum stress in the mice liver: Cross talk between endoplasmic reticulum stress and oxidative stress. Journal of Reports in Pharmaceutical Sciences, 8, 298–302. https://doi.org/10.4103/jrptps.JRPTPS_35_19
Jaswal, V., & Palanivelu, J. C. R. (2018). Effects of the gut microbiota on amygdalin and its use as an anti-cancer therapy: Substantial review on the key components involved in altering dose efficacy and toxicity. Biochemistry and Biophysics Reports, 14, 125–132. https://doi.org/10.1016/j.bbrep.2018.04.008
Song, Z., & Xu, X. (2014). Advanced research on anti-tumor effects of amygdalin. Journal of Cancer Research and Therapeutics, 10, 3–7. https://doi.org/10.4103/0973-1482.139743
Oduwole, I., & Abdelnaser, A. (2020). Amygdalin-therapeutic effects and toxicity. Journal of Biotechnology and Biomedicine, 3(2), 039–049. https://doi.org/10.26502/jbb.2642-91280026
Blaheta, R. A., Nelson, K., Haferkamp, A., & Juengel, E. (2016). Amygdalin, quackery or cure? Phytomedicine, 23, 367–376. https://doi.org/10.1016/j.phymed.2016.02.004
Coates, M. E., & Walker, R. (1992). Interrelationships between the gastrointestinal microflora and nonnutrient components of the diet. Nutrition Research Reviews, 5, 85–96. https://doi.org/10.1079/NRR19920008
Miyake, Y., Suzuki, E., Ohya, S., Fukumoto, S., Hiramitsu, M., Sakaida, K., Osawa, T., & Furuichi, Y. (2006). Lipid-lowering effect of eriocitrin, the main flavonoid in lemon fruit, in rats on a highfat and high-cholesterol diet. Journal of Food Science, 71(9), 633–637. https://doi.org/10.1111/j.1750-3841.2006.00192.x
Nogata, Y., Ohta, H., Ishii, T., & Sekiya, K. (2007). Isolation of eriocitrin (eriodictyol 7-Orutinoside) as an arachidonate lipoxygenase inhibitor from Lumie fruit (Citrus lumia) and its distribution in Citrus species. Journal of the Science of Food and Agriculture, 87(1), 82–89. https://doi.org/10.1002/jsfa.2674
Miyake, Y., Yamamoto, K., & Osawa, T. (1997). Isolation of eriocitrin (eriodictyol 7-rutinoside) from lemon fruit (Citrus limon BURM. f.) and its antioxidative activity. Food Science and Technology International, 3(1), 84–88. https://doi.org/10.3136/fsti9596t9798.3.84
Kwon, E. Y., & Choi, M. S. (2020). Eriocitrin improves adiposity and related metabolic disorders in high-fat diet-induced obese mice. Journal of Medicinal Food, 23(3), 233–241. https://doi.org/10.1089/jmf.2019.4638
Gangadhariah, M., Pardhi, T., Ravilla, J., Chandra, S., & Singh, S. A. (2022). Citrus nutraceutical eriocitrin and its metabolites are partial agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A molecular docking and molecular dynamics study. Journal of Biomolecular Structure &Dynamics, 28, 1–21. https://doi.org/10.1080/07391102.2022.2162127
Yao, L., Liu, W., Bashir, M., Nisar, M. F., & Wan, C. C. (2022). Eriocitrin: A review of pharmacological effects. Biomedicine & Pharmacotherapy, 154, 113563. https://doi.org/10.1016/j.biopha.2022.113563
Yuan, Z., Zhu, M., Wei, X., & Li, X. (2023). Eriocitrin alleviates sevoflurane-induced cytotoxicity in HT22 cells via Nrf2 pathway. Tropical Journal Pharmaceutical Research, 22(2), 259–264. https://doi.org/10.4314/tjpr.v21i2.7
He, J., Zhou, D., & Yan, B. (2020). Eriocitrin alleviates oxidative stress and inflammatory response in cerebral ischemia reperfusion rats by regulating phosphorylation levels of Nrf2/NQO-1/HO-1/NF-κB p65 proteins. Annals of Translational Medicine, 8(12), 757. https://doi.org/10.21037/2Fatm-20-4258
Xu, J., Ma, I., & Fu, P. (2021). Eriocitrin attenuates ischemia reperfusion-induced oxidative stress and inflammation in rats with acute kidney injury by regulating the dualspecificity phosphatase 14 (DUSP14)-mediated Nrf2 and nuclear factor-κB (NF-κB) pathways. Annals Translation of Medicine, 9(4):350. https://doi.org/10.21037/2Fatm-21-337
Duan, X., Hao, Y., Liang, X., Qiu, G., & Xue, W. (2021). Eriocitrin alleviates the arterial occlusionmediated cerebral ischemic-reperfusion injury through the modulation of apoptotic proteins and immune markers in mice. Pharmacognosy Magazine, 17(73), 193–199. https://doi.org/10.4103/pm.pm_577_19
Bassolino, L., Fulvio, F., Pastore, C., Pasini, F., GallinaToschi, T., Filippetti, I., & Paris, R. (2023). When Cannabis sativa L. turns purple: Biosynthesis and accumulation of anthocyanins. Antioxidants, 12, 1393. https://doi.org/10.3390/antiox12071393
Temviriyanukul, P., Sritalahareuthai, V., Jom, K. N., Jongruaysup, B., Tabtimsri, S., Pruesapan, K., Thangsiri, S., Inthachat, W., Siriwan, D., Charoenkiatkul, S., & Suttisansanee, U. (2020). Comparison of phytochemicals, antioxidant, and in vitro anti-Alzheimer properties of twenty-seven Morus spp cultivated in Thailand. Molecules, 25(11), 2600. https://doi.org/10.3390/molecules25112600
Wu, T., Qi, X., Liu, Y., Guo, J., Zhu, R., Chen, W., Zheng, X., & Yuet, T. (2013). Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chemistry, 141(1), 482–487. https://doi.org/10.1016/j.foodchem.2013.03.046
Tyl, C. E., & Bunzel, M. (2012). Antioxidant activity-guided fractionation of blue wheat (UC66049 Triticum aestivum L.). Journal of Agricultural Food Chemistry, 60(3), 731–739. https://doi.org/10.1021/jf203648x
Zhang, J., Hou, X., Ahmad, H., Zhang, H., Zhang, L., & Wang, T. (2014). Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chemistry, 145, 57–65. https://doi.org/10.1016/j.foodchem.2013.08.025
Hatami, M., Mortazavi, M., Baseri, Z., Khani, B., Rahimi, M., & Babaei, S. (2023). Antioxidant compounds in the treatment of Alzheimer’s disease: Natural, hybrid, and synthetic products. Evidence based Complementary and Alternative Medicine, 8056462, 12. https://doi.org/10.1155/2023/8056462
Sharma, S., Sharma, Y. P., Bhardwaj, C., & Mehta, A. (2018). Quantification of amaroswerin and amarogentin in different parts of Swertia chirayita by chromatographic analysis. International Research Journal of Pure Applied Chemistry, 17(4), 1–7. https://doi.org/10.9734/IRJPAC/2018/47081
Niiho, Y., Yamazaki, T., Nakajima, Y., Yamamota, T., Ando, H., & Hirai, Y. (2006). Gastroprotective effects of bitter principles isolated from Gentian root and Swertia herb on experimentally-induced gastric lesions in rats. Journal of Natural Medicines, 60, 82–88. https://doi.org/10.1007/s11418-005-0014-2
Monteiro, A. F. M., Viana, J. O., Nayarisseri, A., Zondegoumba, E. N., Mendonça, J., Junior, F. J. B., Scotti, M. T., & Scotti, L. (2018). Computational studies applied to flavonoids against Alzheimer’s and Parkinson’s diseases. Oxidative Medicine and Cellular Longevity, 30, 7912765. https://doi.org/10.1155/2018/7912765El
Kim, M. S., Young Lee, D., Sung, S. H., & Jeon, K. W. (2018). Anti-cholinesterase activities of hydrolysable tannins and polyhydroxytriterpenoid derivatives from Terminalia chebula Retz. Fruit Records of Natural Prodocts, 12(3), 284–289.
Funding
This work was supported by the King Saud University, Riyadh, Saudi Arabia, under Grant No. RSPD2023R696.
Author information
Authors and Affiliations
Contributions
KK: investigation, formal analysis, molecular docking, and data maintenance. PS: conceptualization, methodology, writing draft, review and editing, and supervision, AM: investigation, formal analysis, critically reviewing and editing the article. TM: data conceptualization, data maintenance, and formal analysis. SK: conceptualization, methodology, fundraising, review and editing, and supervision. VPS: conceptualization, methodology, formal analysis, and review collection. AS: data collection and data interpretation.
Corresponding author
Ethics declarations
Ethical Approval
This research does not require ethical approval.
Consent to Participate
Not applicable.
Consent for Publication
On behalf of the authors, I hereby assign the right of this entire article content to this journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalaimathi, K., Prabhu, S., Ayyanar, M. et al. Unravelling the Untapped Pharmacological Potential of Plant Molecules as Inhibitors of BACE1: In Silico Explorations for Alzheimer’s Disease. Appl Biochem Biotechnol (2023). https://doi.org/10.1007/s12010-023-04803-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04803-4