Abstract
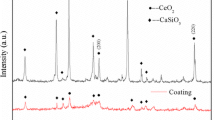
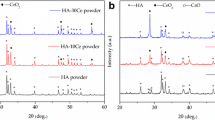
The incidence of bone-related diseases is higher in the elderly population, which greatly affects the patients’ quality of life. Throughout this research, we synthesized a biocomposite nanomaterial of CeO2. The unique structural characteristics of CeO2 nanoparticles (CeO2 NPs) were studied by means of XRD, TEM, and SEM. Nanoparticles of an osteosarcoma cell line (MG-63) were assayed for ALP enzyme levels, key proteins in osteoblasts, and stained with Alizarin Red S to assess the physical properties, bioactivity, and calcium deposition of the osteosarcoma cell line. Moreover, we used H2O2 to construct an oxidative stress model to evaluate the antioxidant activity of CeO2 NPs. Experimental data showed that the CeO2 NPs increased the antioxidant capacity of MG-63 cells and significantly increased alkaline phosphatase activity, calcium deposition, and bone growth as manifested by increased expression of bone differentiation proteins BMP2, OCN, OPN, and type I collagen. Interestingly, RNA interference and functional recovery experiments confirmed that CeO2 NPs enhanced the antioxidant activity of MG-63 cells related to NRF2 signaling. In conclusion, the material is expected to be a potential treatment for bone-related diseases.






Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- XRD:
-
X-ray diffractometer
- SEM:
-
Scanning electron scanning microscope
- TEM:
-
Transmission electron microscope
- DLS:
-
Dynamic light scattering analysis
- NRF2:
-
Red-derived nuclear factor 2-related factor
- DRP1:
-
Dynein-associated protein 1
- BAX:
-
BCL2-associated X
- BCL2:
-
B-cell lymphoma-2
- CAS3:
-
Cancer susceptibility candidate gene 3
References
Rachner, T. D., Khosla, S., & Hofbauer, L. C. (2011). Osteoporosis: now and the future. The Lancet, 377(9773), 1276–1287.
Lei, C., et al. (2023). Advances in materials-based therapeutic strategies against osteoporosis. Biomaterials, 296, 122066.
Kanis, J. A., et al. (2023). The need to distinguish intervention thresholds and diagnostic thresholds in the management of osteoporosis. Osteoporosis International, 34(1), 1–9.
Khinda, R., et al. (2022). Prevalence and predictors of osteoporosis and osteopenia in postmenopausal women of Punjab, India. International Journal of Environmental Research and Public Health, 19(5), 2999.
Siddiqui, J. A., & Partridge, N. C. (2016). Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology, 31(3), 233–245.
Kang, K. S., et al. (2011). Effects of combined mechanical stimulation on the proliferation and differentiation of pre-osteoblasts. Experimental & Molecular Medicine, 43(6), 367–373.
Alborzi, A., et al. (1996). Endochondral and intramembranous fetal bone development: osteoblastic cell proliferation, and expression of alkaline phosphatase, m-twist, and histone H4. Journal of Craniofacial Genetics and Developmental Biology, 16(2), 94–106.
Wehner, C., et al. (2020). Effect of bisphosphonate treatment of titanium surfaces on alkaline phosphatase activity in osteoblasts: a systematic review and meta-analysis. BMC Oral Health, 20, 1–13.
Lim, S. Y., & Bolster, M. B. (2015). Current approaches to osteoporosis treatment. Current Opinion in Rheumatology, 27(3), 216–224.
Lanza, F. L. (2002). Gastrointestinal adverse effects of bisphosphonates: etiology, incidence and prevention. Treatments in Endocrinology, 1, 37–43.
Jeffery, T. C., Chang, A. B., & Conwell, L. S. (2023). Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database of Systematic Reviews, 1(1), CD002010.
Adami, S., & Zamberlan, N. (1996). Adverse effects of bisphosphonates: a comparative review. Drug Safety, 14, 158–170.
Skjødt, M. K., Frost, M., & Abrahamsen, B. (2019). Side effects of drugs for osteoporosis and metastatic bone disease. British Journal of Clinical Pharmacology, 85(6), 1063–1071.
Musson, D. S., et al. (2019). Bovine bone particulates containing bone anabolic factors as a potential xenogenic bone graft substitute. Journal of Orthopaedic Surgery and Research, 14(1), 1–11.
Matussin, S. N., Harunsani, M. H., & Khan, M. M. (2022). CeO2 and CeO2-based nanomaterials for photocatalytic, antioxidant and antimicrobial activities. Journal of Rare Earths, 41(2), 67–181.
Ye, F., et al. (2022). Osteogenic differentiation of mesenchymal stem cells promotes c-Jun-dependent secretion of interleukin 8 and mediates the migration and differentiation of CD4(+) T cells. Stem Cell Research & Therapy, 13(1), 58.
Zhang, F.-L., et al. (2023). Single cell epigenomic and transcriptomic analysis uncovers potential transcription factors regulating mitotic/meiotic switch. Cell Death & Disease, 14(2), 134.
Yu, S., et al. (2020). Chestnut polysaccharides benefit spermatogenesis through improvement in the expression of important genes. Aging (Albany NY), 12(12), 11431.
Zhang, F.-L., et al. (2019). Zearalenone exposure induces the apoptosis of porcine granulosa cells and changes long noncoding RNA expression to promote antiapoptosis by activating the JAK2–STAT3 pathway. Journal of Agricultural and Food Chemistry, 67(43), 12117–12128.
Koncha, R. R., et al. (2021). CCCP-induced mitochondrial dysfunction–characterization and analysis of integrated stress response to cellular signaling and homeostasis. The FEBS Journal, 288(19), 5737–5754.
Heo, S. Y., et al. (2018). Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochemistry and Function, 36(3), 137–146.
Cheng, F., Yang, M., & Yang, R. (2019). MiRNA-365a-3p promotes the progression of osteoporosis by inhibiting osteogenic differentiation via targeting RUNX2. European Review for Medical & Pharmacological Sciences, 23(18), 7766–7774.
Guo, S., et al. (2023). Nanoparticles containing ZnO-TiO2-chitosan and berbamine promote osteoblast differentiation, proliferation, and calcium mineralization in MG63 osteoblasts. Process Biochemistry, 124, 63–70.
Hegedűs, C., et al. (2022). The effect of heat treatment of β-tricalcium phosphate-containing silica-based bioactive aerogels on the cellular metabolism and proliferation of MG63 cells. Biomedicines, 10(3), 662.
Weckstein, S. M., et al. (2017). A retrospective chart analysis with follow-up of cogmed working memory training in children and adolescents with autism spectrum disorder. Medical Science Monitor Basic Research, 23, 336.
Hagar, M. N., et al. (2021). Comparative evaluation of osteogenic differentiation potential of stem cells derived from dental pulp and exfoliated deciduous teeth cultured over granular hydroxyapatite based scaffold. BMC Oral Health, 21(1), 1–13.
Pujari-Palmer, M., et al. (2016). Pyrophosphate stimulates differentiation, matrix gene expression and alkaline phosphatase activity in osteoblasts. PloS one, 11(10), e0163530.
Beck, G. R., et al. (1998). Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. Journal of Cellular Biochemistry, 68(2), 269–280.
Yu, Y., et al. (2018). Osteogenesis potential of different titania nanotubes in oxidative stress microenvironment. Biomaterials, 167, 44–57.
Liguori, I., et al. (2018). Oxidative stress, aging, and diseases. Clinical Interventions in Aging, 13, 757–772.
Sies, H. (2014). Role of metabolic H2O2 generation: redox signaling and oxidative stress. Journal of Biological Chemistry, 289(13), 8735–8741.
Yan, X., et al. (2022). Nrf2 contributes to the benefits of exercise interventions on age-related skeletal muscle disorder via regulating Drp1 stability and mitochondrial fission. Free Radical Biology and Medicine, 178, 59–75.
Sabouny, R., et al. (2017). The Keap1–Nrf2 stress response pathway promotes mitochondrial hyperfusion through degradation of the mitochondrial fission protein Drp1. Antioxidants & Redox Signaling, 27(18), 1447–1459.
Moldoveanu, T., & Czabotar, P. E. (2020). BAX, BAK, and BOK: a coming of age for the BCL-2 family effector proteins. Cold Spring Harbor Perspectives in Biology, 12(4), a036319.
Acknowledgements
We are thankful to Dr. Jun Yan, for critically editing the current manuscript.
Funding
This work is supported by the National Natural Science Foundation of Shandong province (No. ZR202102280280).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to this work.
Corresponding authors
Ethics declarations
Ethical Approval
All experiments in this study have been approved by the Medical Ethics Committee of Liaocheng People’s Hospital with ethics numbers: 2021032 and 2021033.
Consent to Participate
All authors have their consent to participate.
Consent for Publication
All authors have their consent to publish their work.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Wang, Z., Li, X. et al. CeO2 Nanoparticle Bioactive Materials Promote MG-63 Osteogenic Differentiation and Antioxidant Activity Through NRF2 Signaling. Appl Biochem Biotechnol (2023). https://doi.org/10.1007/s12010-023-04766-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04766-6