Abstract
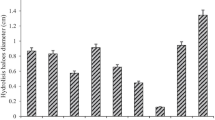
Xylanase production by Streptomyces sp. S1M3I was optimized by response surface methodology (RSM), followed by a partial characterization of these enzymes. Olive pomace was used as a substrate for growing Streptomyces sp. S1M3I in submerged fermentation. Effects of incubation time, pH, temperature, carbon source, nitrogen source, and inoculum size on xylanase production were studied, through the one-factor-at-a-time method. Then, a 33-factorial experimental design with RSM and the Box–Behnken design was investigated for the major influence factors. Maximum xylanase production (11.28 U/mL) was obtained when the strain was grown in mineral medium supplemented with 3% (w/v) of olive pomace powder and 0.3% (w/v) of ammonium sulfate, at a pH 7.4 and an incubation temperature of 40 °C. The xylanases in the supernatant degraded all tested substrates, with higher activity for the low-viscosity wheat arabinoxylan substrate. Two xylanases with close molecular masses were detected by zymogram analysis: Xyl-1 and Xyl-2 with molecular masses of 24.14 kDa and 27 kDa, respectively. The optimization of enzyme production parameters of Streptomyces sp. S1M3I and the characterization of these enzymes are prerequisites to enhancing xylanase production yield, which is crucial for further biotechnological processes.




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Thrash, J.C., & Coates, J.D. (2010). Phylum XVII, Acidobacteria phyl. nov. Bergey's Manual of Systematic Bacteriology. 725‑735. https://doi.org/10.1007/978-0-387-68572-4_6
Boroujeni, M., Das, A., Prachanthi, K., et al. (2012). Enzymatic screening and rendom amplified polymorphic DNA fingerpinting of soil streptomycetes isolated from Wayanad District in Kerala. Indian Journal of Biological Sciences, 12, 1–8. https://doi.org/10.3923/jbs.2012.43.50
Aparicio, J., Zoleica, M., Solá, S., Susana, C., et al. (2015). Safety versatility of Streptomyces sp. M7 to bioremediate soils co-contaminated with Cr (VI) and lindane. Ecotoxicology and Environmental Safety, 116, 34–39. https://doi.org/10.1016/j.ecoenv.2015.02.036
Prakash, D., Nawani, N., Prakash, M., et al. (2013). Actinomycetes : A repertory of green catalysts with a potential revenue resource. BioMed Research International, 2013, 1–8. https://doi.org/10.1155/2013/264020
Irfan, M., Nadeem, M., & Syed, Q. (2014). One-factor-at-a-time ( OFAT ) optimization of xylanase production from Trichoderma viride -IR05 in solid-state fermentation. Journal of Radiation Research and Applied Sciences, 7(3), 317–326. https://doi.org/10.1016/j.jrras.2014.04.004
Garrido, M. M., Piccinni, F. E., Landoni, M., et al. (2022). Insights into the xylan degradation system of Cellulomonas sp. B6: biochemical characterization of rCsXyn10A and rCsAbf62A. Applied Microbiology and Biotechnology, 106(13), 5035–5049. https://doi.org/10.1007/S00253-022-12061-3
Gomathi, D., Muthulakshmi, C., Kumar, D. G., et al. (2012). Submerged fermentation of wheat bran by Aspergillus flavus for production and characterization of carboxy methyl cellulase. Asian Pacific Tropical Biomedical Magazine, 2(1), S67–S73. https://doi.org/10.1016/s2221-1691(12)60132-4
Singhania, R. R., Sukumaran, R. K., Patel, A. K., et al. (2010). Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme and Microbial Technology, 46(7), 541–549. https://doi.org/10.1016/j.enzmictec.2010.03.010
Garai, D., & Kumar, V. (2013). Aqueous two phase extraction of alkaline fungal xylanase in PEG/phosphate system : Optimization by Box – Behnken design approach. Biocatalysis and Agricultural Biotechnology, 2(2), 125–131. https://doi.org/10.1016/j.bcab.2013.03.003
Neifar, M., Jaouani, A., Ayari, A., et al. (2013). Improving the nutritive value of olive cake by solid state cultivation of the medicinal mushroom Fomes fomentarius. Chemosphere, 91, 110–114. https://doi.org/10.1016/j.chemosphere.2012.12.015
Macedo, E. P., Cerqueira, C. L. O., Souza, D. A. J., et al. (2013). Production of cellulose-degrading enzyme on sisal and other agro-industrial residues using a new Brazilian actinobacteria strain Streptomyces sp. SLBA-08. Brazilian Journal of Medical and Biological Research, 30(4), 729–735. https://doi.org/10.1590/s0104-66322013000400005
Pagnanelli, F., Viggi, C. C., & Toro, L. (2010). Development of new composite biosorbents from olive pomace wastes. Applied Surface Science, 256(17), 5492–5497. https://doi.org/10.1016/j.apsusc.2009.12.146
Leite, P., Manuel, J., Venâncio, A., et al. (2016). Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresource Technology, 214, 737–746. https://doi.org/10.1016/j.biortech.2016.05.028
Alam, Z., Muyibi, S. A., & Wahid, R. (2008). Statistical optimization of process conditions for cellulase production by liquid state bioconversion of domestic wastewater sludge. Bioresource Technology, 99, 4709–4716. https://doi.org/10.1016/j.biortech.2007.09.072
Karabegović, I. T., Stojičević, S. S., Veličković, D. T., et al. (2012). Optimization of microwave-assisted extraction of cherry laurel ( Prunus laurocerasus L.) fruit using response surface methodology. Engineering and Technology, 6, 897–902. https://doi.org/10.5281/zenodo.1085596
Medouni-Haroune, L., Zaidi, F., Medouni-Adrar, S., et al. (2017) Selective isolation and screening of Actinobacteria strains producing lignocellulolytic enzymes using olive pomace as substrate. Iranian Journal of Biotechnology,15 (1), 74–77. https://doi.org/10.15171/ijb.1278.
Medouni-Haroune, L., Zaidi, F., Medouni-Adrar, S., et al. (2017). Bioconversion of olive pomace by submerged cultivation of Streptomyces sp. S1M3I. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 88, 1425–1433. https://doi.org/10.1007/s40011-017-0880-x
Lilitchan, S., Tangprawat, C., & Aryusuk, K. (2008). Partial extraction method for the rapid analysis of total lipids and c-oryzanol contents in rice bran. Food Chemistry, 106, 752–759. https://doi.org/10.1016/j.foodchem.2007.06.052
Tuncer, M., Ball, A. S., Rob, A., et al. (1999). Optimization of extracellular lignocellulolytic enzyme production by a thermophilic actinomycete Thermomonospora fusca BD25. Enzyme and Microbial Technology, 25(1–2), 38–47. https://doi.org/10.1016/S0141-0229(99)00012-5
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685. https://doi.org/10.1038/227680a0
Dhillon, A., Gupta, J. K., & Khanna, S. (2000). Enhanced production, purification and characterisation of a novel cellulase-poor thermostable, alkalitolerant xylanase from Bacillus circulans AB 16. Process Biochemistry, 35, 849–856. https://doi.org/10.1016/s0032-9592(99)00152-1
Danso, B., Ali, S. S., Xie, R., et al. (2022). Valorisation of wheat straw and bioethanol production by a novel xylanase and cellulase-producing Streptomyces strain isolated from the wood-feeding termite. Microcerotermes species. Fuel, 310, 122333. https://doi.org/10.1016/j.fuel.2021.122333
Xiuting, L., Baoguo, S., Jin, Z., et al. (2011). Production and improved bleaching abilities of a thermostable xylanase from a newly isolated Streptomyces chartreusis strain. African Journal of Biotechnology, 10(64), 14132–14142. https://doi.org/10.5897/AJB10.2360
Techapun, C., Charoenrat, T., Watanabe, M., et al. (2002). Optimization of thermostable and alkaline-tolerant cellulase-free xylanase production from agricultural waste by thermotolerant Streptomyces sp. Ab106, using the central composite experimental design. Biochemical Engineering Journal, 12, 99–105. https://doi.org/10.1016/s1369-703x(02)00047-5
Khangkhachit, W., Suyotha, W., Leamdum, C., et al. (2021). Production of thermostable xylanase using Streptomyces thermocarboxydus ME742 and application in enzymatic conversion of xylan from oil palm empty fruit bunch to xylooligosaccharides. Biocatalysis and Agricultural Biotechnology, 37, 102180. https://doi.org/10.1016/j.bcab.2021.102180
Romero, P., Lussier, M., Véronneau, S., et al. (1999). Mnt2p and Mnt3p of Saccharomyces cerevisiae are members of the Mnn1p family of alpha-1,3-mannosyltransferases responsible for adding the terminal mannose residues of O-linked oligosaccharides. Glycobiology, 9(10), 1045–1051. https://doi.org/10.1093/glycob/9.10.1045
Bajaj, B. K., & Singh, N. P. (2010). Production of xylanase from an alkali tolerant Streptomyces sp. 7b under solid-state fermentation, its purification, and characterization. Applied Biochemistry and Biotechnology, 162(6), 1804–1818. https://doi.org/10.1007/s12010-010-8960-x
Miyanaga, K., & Unno, H. (2011) Reaction kinetics, and stoichiometry. Comprehensive Biotechnology, 33–46. https://doi.org/10.1016/b978-0-08-088504-9.00085-4.
Sharma, H. K., Xu, C., & Qin, W. (2021). Isolation of bacterial strain with xylanase and xylose/glucose isomerase (GI) activity and whole cell immobilization for improved enzyme production. Waste Biomass Valorization, 12, 833–845. https://doi.org/10.1007/s12649-020-01013-5
Adhi, T. P., Korus, R. A., & Crawford, D. L. (1989). Production of major extracellular enzymes during lignocellulose degradation by two streptomycetes in agitated submerged culture. Applied and Environmental Microbiology, 55(5), 1165–1168. https://doi.org/10.1128/aem.55.5.1165-1168.1989
Boucherba, N., Benallaoua, S., Copinet, E., et al. (2011). Production and partial characterization of xylanase produced by Jonesia denitrificans isolated in Algerian soil. Process Biochemistry, 46(2), 519–525. https://doi.org/10.1016/j.procbio.2010.10.003
McCarthy, A. (1987). Lignocellulose degrading actinomycetes. FEMS Microbiology Reviews, 46, 145–163.
Vance, E. D., & Chapin, F. S. (2001). Substrate limitations to microbial activity in taiga forest floors. Soil Biology and Biochemistry, 33, 173–188.
Naidu, G., & Panda, T. (1998). Production of pectolytic enzymes e a review. Bioprocess Engineering, 19, 355–361. https://doi.org/10.1007/pl00009023
Salhi, M.O. (2004) Valorisation de sous-produits et déchets lignocellulosiques par culture de microorganismes cellulolytiques.Dissertation, Insitut national agronomique El-harrache.
Kumar, A., Gupta, R., Shrivastava, B., et al. (2012). Xylanase production from an alkalophilic actinomycete isolate Streptomyces sp. RCK-2010, its characterization and application in saccharification of second generation biomass. Journal of Molecular Catalysis B: Enzymatic, 74(3–4), 170–177. https://doi.org/10.1016/j.molcatb.2011.10.001
Nascimento, R. P., Coelho, R. R. R., Marques, S., et al. (2002). Production and partial characterisation of xylanase from Streptomyces sp. strain AMT-3 isolated from Brazilian cerrado soil. Enzyme and Microbial Technology, 31, 549–555. https://doi.org/10.1016/S0141-0229(02)00150-3
Battestin, V., & Macedo, G. A. (2007). Effects of temperature, pH and additives on the activity of tannase produced by Paecilomyces variotii. Electronic Journal of Biotechnology, 10(2), 191–199. https://doi.org/10.2225/vol10-issue2-fulltext-9
Haaland, P. D. (1989) Experimental design in biotechnology. New York.
Sharma, P., & Bajaj, B. K. (2005). Production and partial characterization of alkali-tolerant xylanase from an alkalophilic Streptomyces sp. CD3. Journal of Scientific & Industrial Research, 64, 688–697.
Lafond, M., Tauzin, A., Desseaux, V., et al. (2011). GH10 xylanase D from Penicillium funiculosum : Biochemical studies and xylooligosaccharide production. Microbial Cell Factories, 10(1), 1–8. https://doi.org/10.1186/1475-2859-10-20
Kulkarni, N., Shendye, A., & Rao, M. (1999). Molecular and biotechnological aspects of xylanases. FEMS Microbiology Reviews, 23(4), 411–456. https://doi.org/10.1111/j.1574-6976.1999.tb00407.x
Taibi, Z., Saoudi, B., Boudelaa, M., et al. (2012). Purification and biochemical characterization of a highly thermostable xylanase from Actinomadura sp. strain Cpt20 isolated from poultry compost. Applied Biochemistry and Biotechnology, 166, 663–679. https://doi.org/10.1007/s12010-011-9457-y
Chi, W., Park, D. Y., & Chang, Y. (2012). A novel alkaliphilic xylanase from the newly isolated mesophilic Bacillus sp. MX47: Production, purification and characterization. Applied Biochemistry and Biotechnology, 168, 899–909. https://doi.org/10.1007/s12010-012-9828-z
Acknowledgements
We thank Professor S. Roussos and V. Desseaux for the training in the IMBE and ISM2, Marseille (France).
Funding
The research was supported by the Faculty of Nature and Life Sciences, University of Bejaia, Algeria.
Author information
Authors and Affiliations
Contributions
Lamia Medouni-Haroune: conceptualization, experiments, and writing original draft; Sonia Medouni-Adrar: methodology and data curation; Aicha Asma Houfani: reviewing and editing; Cilia Bouiche and Zahra Azzouz: polished the paper; Sevastianos Roussos: provides research guidance; Véronique Desseaux: analytical methodology; Khodir Madani and Mouloud Kecha: supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Medouni-Haroune, L., Medouni-Adrar, S., Houfani, A.A. et al. Statistical Optimization and Partial Characterization of Xylanases Produced by Streptomyces sp. S1M3I Using Olive Pomace as a Fermentation Substrate. Appl Biochem Biotechnol 196, 2012–2030 (2024). https://doi.org/10.1007/s12010-023-04660-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04660-1