Abstract
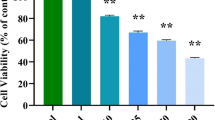
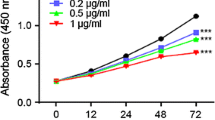
Colon cancer is the most prevalent cancer and causes the highest cancer-associated mortality in both men and women globally. It has a high incidence and fatality rate, which places a significant burden on the healthcare system. The current work was performed to understand the beneficial roles of nerolidol on the viability and cytotoxic mechanisms in the colon cancer HCT-116 cells. The MTT cytotoxicity assay was done to investigate the effect of nerolidol at different doses (5–100 µM) on the HCT-116 cell viability. The impacts of nerolidol on ROS accumulation and apoptosis were investigated using DCFH-DA, DAPI, and dual staining assays, respectively. The flow cytometry analysis was performed to study the influence of nerolidol on the cell cycle arrest in the HCT-116 cells. The outcomes of the MTT assay demonstrated that nerolidol at different doses (5–100 µM) substantially inhibited the HCT-116 cell viability with an IC50 level of 25 µM. The treatment with nerolidol appreciably boosted the ROS level in the HCT-116 cells. The findings of DAPI and dual staining revealed higher apoptotic incidences in the nerolidol-exposed HCT-116 cells, which supports its ability to stimulate apoptosis. The flow cytometry analysis demonstrated the considerable inhibition in cell cycle at the G0/G1 phase in the nerolidol-exposed HCT-116 cells. Our research showed that nerolidol can inhibit the cell cycle, increase ROS accumulation, and activate apoptosis in HCT-116 cells. In light of this, it may prove to be a potent and salutary candidate to treat colon cancer.





Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- DMOS:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- ROS:
-
Reactive oxygen species
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DCFH-DA:
-
2′-7′-Dichlorodihydrofluorescein diacetate
References
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer statistics, 2021. CA: A Cancer Journal for Clinicians, 71, 7–33.
Pamudurthy, V., Lodhia, N., Konda, V. J., editors. (2020). Advances in endoscopy for colorectal polyp detection and classification. Baylor University Medical Center Proceedings, Taylor & Francis.
Vos, T., Lim, S. S., Abbafati, C., Abbas, K. M., Abbasi, M., Abbasifard, M., et al. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet, 396(10258), 1204–1222.
Liu, K. C., Shih, T. Y., Kuo, C. L., Ma, Y. S., Yang, J. L., Wu, P. P., Huang, Y. P., Lai, K. C., & Chung, J. G. (2016). Sulforaphane induces cell death through G2/M phase arrest and triggers apoptosis in HCT 116 human colon cancer cells. American Journal of Chinese Medicine, 44, 1289–1310.
Xie, Y. H., Chen, Y. X., & Fang, J. Y. (2020). Comprehensive review of targeted therapy for colorectal cancer. Signal Transduction and Targeted Therapy, 5, 22.
Chang, J., Jung, H. J., Jeong, S. H., Kim, H. K., Han, J., & Kwon, H. J. (2014). A mutation in the mitochondrial protein UQCRB promotes angiogenesis through the generation of mitochondrial reactive oxygen species. Biochemical and Biophysical Research Communications, 455, 290–297.
Goldar, S., Khaniani, M., Derakhshan, S., & Baradaran, B. (2015). Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pacific Journal of Cancer Prevention, 16, 2129–2144.
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., Jr., & Kinzler, K. W. (2013). Cancer genome landscapes. Science, 339(6127), 1546–1558.
Sharma, A., Boise, L. H., & Shanmugam, M. (2019). Cancer metabolism and the evasion of apoptotic cell death. Cancers (Basel), 11(8), 1144.
Abaza, A., Vasavada, A. M., Sadhu, A., Valencia, C., Fatima, H., Nwankwo, I., Anam, M., Maharjan, S., Amjad, Z., & Khan, S. (2022). A systematic review of apoptosis in correlation with cancer: Should apoptosis be the ultimate target for cancer treatment? Cureus, 14(8), e28496.
Morawska, K., Goirand, F., Marceau, L., Devaux, M., Cueff, A., Bertaut, A., Vincent, J., Bengrine-Lefevre, L., Ghiringhelli, F., & Schmitt, A. (2018). 5-FU therapeutic drug monitoring as a valuable option to reduce toxicity in patients with gastrointestinal cancer. Oncotarget, 9, 11559–11571.
Aoullay, Z., Slaoui, M., Razine, R., Er-Raki, A., Meddah, B., & Cherrah, Y. (2020). Therapeutic characteristics, chemotherapy-related toxicities and survivorship in colorectal cancer patients. Ethiopian Journal of Health Sciences, 30(1), 65–74.
Millimouno, F. M., Dong, J., Yang, L., Li, J., & Li, X. (2014). Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prevention Research, 7, 1081–1107.
Zhou, Y., Zeng, L., Liu, X., Gui, J., Mei, X., Fu, X., Dong, F., Tang, J., Zhang, L., & Yang, Z. (2017). Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chemistry, 231, 78–86.
Chan, W.-K., Tan, L. T., Chan, K.-G., Lee, L.-H., & Goh, B.-H. (2016). Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules, 21, 529.
De Carvalho, R. B. F., De Almeida, A. A. C., Campelo, N. B., Lellis, D., & Nunes, L. C. C. (2018). Nerolidol and its pharmacological application in treating neurodegenerative diseases: A review. Recent Patents on Biotechnology, 12, 158–168.
Javed, H., Azimullah, S., Abul Khair, S. B., Ojha, S., & Haque, M. E. (2016). Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neuroscience, 17, 58.
Baldissera, M. D., Souza, C. F., Grando, T. H., Dolci, G. S., Cossetin, L. F., Moreira, K. L., et al. (2017). Nerolidol-loaded nanospheres prevent hepatic oxidative stress of mice infected by Trypanosoma evansi. Parasitology, 144, 148–157.
Iqubal, A., Sumit, S., Ansari, M. A., Najmi, A. K., Syed, M. A., Ali, J., et al. (2019). Nerolidol attenuates cyclophosphamide-induced cardiac inflammation, apoptosis and fibrosis in Swiss albino mice. European Journal of Pharmacology, 863, 172666.
Thapa, D., Richardson, A. J., Zweifel, B., Wallace, R. J., & Gratz, S. W. (2019). Genoprotective effects of essential oil compounds against oxidative and methylated DNA damage in human colon cancer cells. Journal of Food Science, 84, 1979–1985.
Fonseca, D. V., Salgado, P. R., de Carvalho, F. L., Salvadori, M. G., Penha, A. R., Leite, F. C., et al. (2016). Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundamental & Clinical Pharmacology, 30, 14–22.
Ferreira, F. M., Palmeira, C. M., Oliveira, M. M., Santos, D., Simoes, A. M., Rocha, S. M., Coimbra, M. A., & Peixoto, F. (2012). Nerolidol effects on mitochondrial and cellular energetics. Toxicology in Vitro, 26, 189–196.
Hanusova, V., Caltova, K., Svobodova, H., Ambroz, M., Skarka, A., Murinova, N., Kralova, V., Tomsik, P., & Skalova, L. (2017). The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumorbearing mice. Biomedicine & Pharmacotherapy, 95, 828–836.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249.
Grunwald, V., Karch, A., Schuler, M., Schoffski, P., Kopp, H. G., Bauer, S., et al. (2020). Randomized comparison of pazopanib and doxorubicin as first-line treatment in patients with metastatic soft tissue sarcoma age 60 years or older: Results of a German Intergroup Study. Journal of Clinical Oncology, 38, 3555–3564.
Pietzsch, S., Wohlan, K., Thackeray, J. T., Heimerl, M., Schuchardt, S., Scherr, M., et al. (2021). Anthracycline-free tumor elimination in mice leads to functional and molecular cardiac recovery from cancer-induced alterations in contrast to long-lasting doxorubicin treatment effects. Basic Research in Cardiology, 116, 61.
Fouad, Y. A., & Aanei, C. (2017). Revisiting the hallmarks of cancer. American Journal of Cancer Research, 7(5), 1016–1036.
Lee, S. O., Joo, S. H., Kwak, A. W., Lee, M. H., Seo, J. H., Cho, S. S., Yoon, G., Chae, J. I., & Shim, J. H. (2021). Podophyllotoxin induces ROS-mediated apoptosis and cell cycle arrest in human colorectal cancer cells via p38 MAPK signaling. Biomol Ther (Seoul), 29(6), 658–666.
Porporato, P. E., Filigheddu, N., Bravo-San Pedro, J. M., Kroemer, G., & Galluzzi, L. (2018). Mitochondrial metabolism and cancer. Cell Research, 28, 265–280.
Badrinath, N., & Yoo, S. Y. (2018). Mitochondria in cancer: In the aspects of tumorigenesis and targeted therapy. Carcinogenesis, 39, 1419–1430.
Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., Castoria, G., & Migliaccio, A. (2020). ROS in cancer therapy: The bright side of the moon. Experimental & Molecular Medicine, 52, 192–203.
Liu, Y., Shi, C., He, Z., Zhu, F., Wang, M., He, R., et al. (2021). Inhibition of PI3K/AKT signaling via ROS regulation is involved in Rhein-induced apoptosis and enhancement of oxaliplatin sensitivity in pancreatic cancer cells. International Journal of Biological Sciences, 17, 589–602.
Mi, Y., Xiao, C., Du, Q., Wu, W., Qi, G., & Liu, X. (2016). Momordin Ic couples apoptosis with autophagy in human hepatoblastoma cancer cells by reactive oxygen species (ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radical Biology & Medicine, 90, 230–242.
Lin, H. L., Chen, P. R., Mao, C. C., Zheng, W. E., & Wang, J. Q. (2021). Ferruginol-induced apoptosis in Human Colon Cancer Cells (HCT-116) through the mitochondria-mediated apoptotic pathway. Pharmacognosy Magazine, 17, 244–249.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674.
Wong, R. S. (2011). Apoptosis in cancer: From pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research, 30, 87.
Valentini, E., D’Aguanno, S., Di Martile, M., Montesano, C., Ferraresi, V., Patsilinakos, A., Sabatino, M., Antonini, L., Chiacchiarini, M., Valente, S., Mai, A., Colotti, G., Ragno, R., Trisciuoglio, D., & Bufalo, D. D. (2022). Targeting the anti-apoptotic Bcl-2 family proteins: Machine learning virtual screening and biological evaluation of new small molecules. Theranostics., 12, 2427–2444.
Xiang, L., He, B., Liu, Q., Hu, D., Liao, W., Li, R., Peng, X., Wang, Q., & Zhao, G. (2020). Antitumor effects of curcumin on the proliferation, migration and apoptosis of human colorectal carcinoma HCT-116 cells. Oncology Reports, 44, 1997–2008.
Neophytou, C. M., Trougakos, I. P., Erin, N., & Papageorgis, P. (2021). Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers, 13, 4363.
Elmore, S. (2017). Apoptosis: A review of programmed cell death. Toxicologic Pathology, 35(4), 495–516.
Dangroo, N. A., Singh, J., Ratha, S. K., Gupta, N., Qayum, A., Singh, S., & Sangwana, P. L. (2017). A convergent synthesis of novel alkyne–azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids, 123, 1–12.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Afrin, S., Giampieri, F., Gasparrini, M., Forbes-Hernandez, T. Y., Varela-López, A., Quiles, J. L., et al. (2016). Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules, 21, 169.
Fulda, S., Galluzzi, L., & Kroemer, G. (2010). Targeting mitochondria for cancer therapy. Nature Reviews Drug Discovery, 9, 447–464.
Bajpai, V. K., Khan, I., Shukla, S., Kang, S. M., Aziz, F., Tripathi, K. M., Saini, D., Cho, H. J., Heo, N. S., Sonkar, S. K., et al. (2020). Multifunctional N-P-doped carbon dots for regulation of apoptosis and autophagy in B16F10 melanoma cancer cells and in vitro imaging applications. Theranostic, 10, 7841–7856.
Al-Zharani, M., Nasr, F. A., Alqahtani, A. S., Cordero, M. A. W., Alotaibi, A. A., & Bepari, A. (2021). In vitro cytotoxic evaluation and apoptotic effects of Datura innoxia grown in Saudi Arabia and phytochemical analysis. Applied Sciences, 11, 2864.
Zhaojun, C., Lulin, T., Xin, F., Abdel-nasser, S., Zunguo, L., & Xiong, L. (2022). Hydroxy-g-sanshool from Zanthoxylum bungeanum (prickly ash) induces apoptosis of human colorectal cancer cell by activating P53 and Caspase 8. Frontiers in Nutrition, 9, 914638.
Kwon, M., Oh, T., Jang, M., Kim, G. H., Kim, J. H., Ryu, H. W., Oh, S. R., Jang, J. H., Ahn, J. S., & Ko, S. K. (2022). Kurarinone induced p53-independent G0/G1 cell cycle arrest by degradation of K-RAS via WDR76 in human colorectal cancer cells. European Journal of Pharmacology, 15(923), 174938.
Funding
This project was supported by Researchers Supporting Project number (RSP2023R383), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
The authors contributed equally.
Corresponding author
Ethics declarations
Ethics Approval
All work has been done under the guidelines of Institutional Ethics Committee.
Consent to Participate
All authors have their consent to participate.
Consent for Publication
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Chinnathambi, A., Alharbi, S.A. et al. Nerolidol, Bioactive Compound Suppress Growth of HCT-116 Colorectal Cancer Cells Through Cell Cycle Arrest and Induction of Apoptosis. Appl Biochem Biotechnol 196, 1365–1375 (2024). https://doi.org/10.1007/s12010-023-04612-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04612-9