Abstract
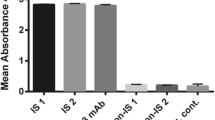
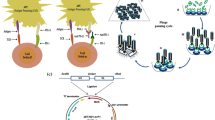
The pentaspan transmembrane glycoprotein CD133, prominin-1, is expressed in cancer stem cells in many tumors and is promising as a novel target for the delivery of cytotoxic drugs to cancer-initiating cells. In this study, we prepared a mouse library of single-chain variable fragment (scFv) antibodies using mRNAs isolated from mice immunized with the third extracellular domain of a recombinant CD133 (D-EC3). First, the scFvs were directly exposed to D-EC3 to select a new specific scFv with high affinity against CD133 using the ribosome display method. Then, the selected scFv was characterized by the indirect enzyme-linked immunosorbent assay (ELISA), immunocytochemistry (ICC), and in silico analyses included molecular docking and molecular dynamics simulations. Based on ELISA results, scFv 2 had a higher affinity for recombinant CD133, and it was considered for further analysis. Next, the immunocytochemistry and flow cytometry experiments confirmed that the obtained scFv could bind to the CD133 expressing HT-29 cells. Furthermore, the results of in silico analysis verified the ability of the scFv 2 antibody to bind and detect the D-EC3 antigen through key residues employed in antigen–antibody interactions. Our results suggest that ribosome display could be applied as a rapid and valid method for isolation of scFv with high affinity and specificity. Also, studying the mechanism of interaction between CD133’s scFv and D-EC3 with two approaches of experimental and in silico analysis has potential importance for the design and development of antibody with improved properties.










Similar content being viewed by others
Data Availability
The datasets generated during the current study are available in the GenBank accession number: LC655309.
References
Carr, B. J., Stanar, P., & Moritz, O. L. (2020). Genetically-modified X. laevisindicate distinct roles for prominin-1 and photoreceptor cadherin in outer segment morphogenesis and retinal dystrophy. bioRxiv, 24, 2020–2028.
Ghani, S., Bahrami, S., Rafiee, B., Eyvazi, S., Yarian, F., & Ahangarzadeh, S. (2020). Recent developments in antibody derivatives against colorectal cancer; A review. Life Sciences, 265, 118791.
Zella, M. A. K., Sebastião, A. P. M., Collaço, L. M., Ogata, D. C., Cecchetti, G., & Bartolomei, I. J. P. (2020). Prognostic significance of CD133 and ABCB5 expression in papillary thyroid carcinoma. European Journal of Histochemistry: EJH, 64(4), 3143.
Zhou, G., Bae, S. D. W., Nguyen, R., Huo, X., Han, S., Zhang, Z., et al. (2021). An aptamer-based drug delivery agent (CD133-apt-Dox) selectively and effectively kills liver cancer stem-like cells. Cancer Letters, 501, 124–132.
Aghajani, M., Mokhtarzadeh, A., Aghebati-Maleki, L., Mansoori, B., Mohammadi, A., Safaei, S., et al. (2020). CD133 suppression increases the sensitivity of prostate cancer cells to paclitaxel. Molecular Biology Reports, 47(5), 3691–3703.
Yamashita, N., Oyama, T., So, T., Miyata, T., Yoshimatsu, T., Nakano, R., et al. (2021). Association between CD133 expression and prognosis in human lung adenocarcinoma. Anticancer Research, 41(2), 905–910.
Yuan, Z., Liang, X., Zhan, Y., Wang, Z., Xu, J., Qiu, Y., et al. (2020). Targeting CD133 reverses drug-resistance via the AKT/NF-κB/MDR1 pathway in colorectal cancer. British Journal of Cancer, 122(9), 1342–1353.
Ghani, S., Deravi, N., Pirzadeh, M., Rafiee, B., Gatabi, Z. R., & Bandehpour, M. (2021). Antibody fragment and targeted colorectal cancer therapy: A global systematic review. Current Pharmaceutical Biotechnology, 23(8), 1061–1071.
Smith, L. M., Nesterova, A., Ryan, M. C., Duniho, S., Jonas, M., Anderson, M., et al. (2008). CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. British Journal of Cancer, 99(1), 100–109.
Ohlfest, J. R., Zellmer, D. M., Panyam, J., Swaminathan, S. K., Oh, S., Waldron, N. N., et al. (2013). Immunotoxin targeting CD133 + breast carcinoma cells. Drug Delivery and Translational Research, 3, 195–204.
Zamani Dehkordi, N., Zia Jahromi, N., & Sazgar, H. (2019). Comparison and comparison of CD133 gene expression in healthy individuals and people with leukemia. New Cellular and Molecular Biotechnology Journal, 9(36), 43–52.
Wang, Z., Sun, M., Li, W., Fan, L., Zhou, Y., & Hu, Z. (2020). A novel CD133-and EpCAM-targeted liposome with redox-responsive properties capable of synergistically eliminating liver cancer stem cells. Frontiers in Chemistry, 8, 649.
Tan, H., Hou, N., Liu, Y., Liu, B., Cao, W., Zheng, D. (2020). CD133 antibody targeted delivery of gold nanostars loading IR820 and docetaxel for multimodal imaging and near-infrared photodynamic/photothermal/chemotherapy against castration resistant prostate cancer. Nanomedicine: Nanotechnology, Biology and Medicine, 27, 102192.
Bidlingmaier, S., Zhu, X., & Liu, B. (2008). The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. Journal of Molecular Medicine, 86(9), 1025.
Glumac, P. M., Forster, C. L., Zhou, H., Murugan, P., Gupta, S., & LeBeau, A. M. (2018). The identification of a novel antibody for CD133 using human antibody phage display. The Prostate, 78(13), 981–991.
Glumac, P. M., & LeBeau, A. M. (2018). The role of CD133 in cancer: A concise review. Clinical and Translational Medicine, 7, 1–14.
Kemper, K., Sprick, M. R., de Bree, M., Scopelliti, A., Vermeulen, L., Hoek, M., et al. (2010). The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Research, 70(2), 719–729.
Zambrano, N., Froechlich, G., Lazarevic, D., Passariello, M., Nicosia, A., De Lorenzo, C., et al. (2022). High-throughput monoclonal antibody discovery from phage libraries: Challenging the current preclinical pipeline to keep the pace with the increasing mAb demand. Cancers, 14(5), 1325.
Lim, M. S. H., Ohtsuki, T., Takenaka, F., Kobayashi, K., Akehi, M., Uji, H., et al. (2021). A novel 89Zr-Labeled DDS device utilizing human IgG variant (ScFv):“Lactosome” nanoparticle-based theranostics for PET imaging and targeted therapy. Life, 11(2), 158.
Muñoz-López, P., Ribas-Aparicio, R. M., Becerra-Báez, E. I., Fraga-Pérez, K., Flores-Martínez, L. F., Mateos-Chávez, A. A., et al. (2022). Single-chain Fragment Variable: Recent progress in Cancer diagnosis and therapy. Cancers, 14(17), 4206.
Mattheakis, L. C., Bhatt, R. R., & Dower, W. J. (1994). An in vitro polysome display system for identifying ligands from very large peptide libraries. Proceedings of the National Academy of Sciences, 91(19), 9022–6.
Kunamneni, A., Ogaugwu, C., Bradfute, S., & Durvasula, R. (2020). Ribosome display technology: applications in disease diagnosis and control. Antibodies, 9(3), 28.
Li, R., Kang, G., Hu, M., & Huang, H. (2019). Ribosome display: A potent display technology used for selecting and evolving specific binders with desired properties. Molecular Biotechnology, 61, 60–71.
Ghani, S., Yarian, F., Bandehpour, M., & Kazemi, B. (2021). An in-silico approach and experimental analysis combination: two strategies for selecting the third Extracellular Domain (D-EC3) of human CD133 marker as a target for detection of Cancer Stem cells. Iranian Journal of Pharmaceutical Research, 20(4), 80–91.
Azizi, A., Arora, A., Markiv, A., Lampe, D. J., Miller, T. A., & Kang, A. S. (2012). Ribosome display of combinatorial antibody libraries derived from mice immunized with heat-killed Xylella fastidiosa and the selection of MopB-specific single-chain antibodies. Applied and Environmental Microbiology, 78(8), 2638–2647.
Luo, Y., & Xia, Y. (2012). Selection of single-chain variable fragment antibodies against fenitrothion by ribosome display. Analytical Biochemistry, 421(1), 130–137.
Sanger, F., Nicklen, S., & Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences, 74(12), 5463–7.
Sankian, M., Yousefi, M., Pazouki, N., & Varasteh, A. (2007). One-step purification of histidine‐tagged profilin with high purity and yield by using metal precipitation. Biotechnology and Applied Biochemistry, 47(4), 185–189.
Beatty, J. D., Beatty, B. G., & Vlahos, W. G. (1987). Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. Journal of Immunological Methods, 100(1–2), 173–179.
Walter, D., Satheesha, S., Albrecht, P., Bornhauser, B. C., D’Alessandro, V., Oesch, S. M., et al. (2011). CD133 positive embryonal rhabdomyosarcoma stem-like cell population is enriched in rhabdospheres. PLoS One, 6(5), e19506.
Elsaba, T. M., Martinez-Pomares, L., Robins, A. R., Crook, S., Seth, R., Jackson, D., et al. (2010). The stem cell marker CD133 associates with enhanced colony formation and cell motility in colorectal cancer. PLoS One, 5(5), e10714.
Brenke, R., Hall, D. R., Chuang, G. Y., Comeau, S. R., Bohnuud, T., Beglov, D., et al. (2012). Application of asymmetric statistical potentials to antibody–protein docking. Bioinformatics, 28(20), 2608–2614.
Hess, B., Kutzner, C., Van Der Spoel, D., & Lindahl, E. (2008). GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. Journal of Chemical Theory and Computation, 4(3), 435–447.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nature Reviews Cancer, 8(10), 755–768.
Medema, J. P. (2013). Cancer stem cells: The challenges ahead. Nature Cell Biology, 15(4), 338–344.
Lee, H. J., Do You, D., Choi, D. W., Choi, Y. S., Kim, S. J., Won, Y. S., et al. (2011). Significance of CD133 as a cancer stem cell markers focusing on the tumorigenicity of pancreatic cancer cell lines. Journal of the Korean Surgical Society, 81(4), 263–270.
Hsin, I. L., Chiu, L. Y., Ou, C. C., Wu, W. J., Sheu, G. T., & Ko, J. L. (2020). CD133 inhibition via autophagic degradation in pemetrexed-resistant lung cancer cells by GMI, a fungal immunomodulatory protein from Ganoderma microsporum. British Journal of Cancer, 123(3), 449–458.
Blancas-Mosqueda, M., Zapata-Benavides, P., Zamora-Ávila, D., Saavedra-Alonso, S., Manilla-Muñoz, E., Franco-Molina, M., et al. (2012). CD133 antisense suppresses cancer cell growth and increases sensitivity to cisplatin in vitro. Experimental and Therapeutic Medicine, 4(5), 901–905.
Asadzadeh, Z., Mansoori, B., Mohammadi, A., Kazemi, T., Mokhtarzadeh, A., Shanehbandi, D., et al. (2021). The combination effect of Prominin1 (CD133) suppression and oxaliplatin treatment in colorectal cancer therapy. Biomedicine & Pharmacotherapy, 137, 111364.
Chen, W., Li, F., Xue, Z. M., & Wu, H. R. (2010). Anti-human CD133 monoclonal antibody that could inhibit the proliferation of colorectal cancer cells. Hybridoma (2005), 29(4), 305–310.
Swaminathan, S. K., Olin, M. R., Forster, C. L., Santa Cruz, K. S., Panyam, J., & Ohlfest, J. R. (2010). Identification of a novel monoclonal antibody recognizing CD133. Journal of Immunological Methods, 361(1–2), 110–115.
Itai, S., Fujii, Y., Nakamura, T., Chang, Y. W., Yanaka, M., Saidoh, N., et al. (2017). Establishment of CMab-43, a sensitive and specific anti-CD133 monoclonal antibody, for immunohistochemistry. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 36(5), 231–235.
Kato, Y., Ohishi, T., Yamada, S., Itai, S., Furusawa, Y., Sano, M., et al. (2019). Anti-CD133 monoclonal antibody CMab-43 exerts antitumor activity in a mouse xenograft model of colon cancer. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 38(2), 75–78.
Xia, J., Zhang, Y., Qian, J., Zhu, X., Zhang, Y., Zhang, J., et al. (2013). Isolation, identification and expression of specific human CD133 antibodies. Scientific Reports, 3(1), 1–9.
Swaminathan, S. K., Niu, L., Waldron, N., Kalscheuer, S., Zellmer, D. M., Olin, M. R., et al. (2013). Identification and characterization of a novel scFv recognizing human and mouse CD133. Drug Delivery and Translational Research, 3(2), 143–151.
Waldron, N. N., Kaufman, D. S., Oh, S., Inde, Z., Hexum, M. K., Ohlfest, J. R., et al. (2011). Targeting tumor-initiating Cancer cells with dCD133KDEL shows impressive Tumor Reductions in a Xenotransplant Model of Human Head and Neck CancerTargeting CD133-Expressing Cancer cells. Molecular Cancer Therapeutics, 10(10), 1829–1838.
Skubitz, A. P., Taras, E. P., Boylan, K. L., Waldron, N. N., Oh, S., Panoskaltsis-Mortari, A., et al. (2013). Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecologic Oncology, 130(3), 579–587.
Olsen, C. E., Cheung, L. H., Weyergang, A., Berg, K., Vallera, D. A., Rosenblum, M. G., et al. (2019). Design, characterization, and evaluation of scFvCD133/rGelonin: A CD133-targeting recombinant immunotoxin for use in combination with photochemical internalization. Journal of Clinical Medicine, 9(1), 68.
Kaplon, H., Muralidharan, M., Schneider, Z., & Reichert, J. M. (Eds.). (2020). Antibodies to watch in 2020. MAbs.
Ahmad, Z. A., Yeap, S. K., Ali, A. M., Ho, W. Y., Alitheen, N. B. M., & Hamid, M. (2012). scFv antibody: Principles and clinical application. Clinical and Developmental Immunology, Article ID 980250, pp. 15.
Kowalski, M., Entwistle, J., Cizeau, J., Niforos, D., Loewen, S., Chapman, W., et al. (2010). A phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCGrefractory and BCG-intolerant patients. Drug Design Development and Therapy, 4, 313.
Wang, Y. P., Liu, I. J., Chung, M. J., & Wu, H. C. (2020). Novel anti-EGFR scFv human antibody-conjugated immunoliposomes enhance chemotherapeutic efficacy in squamous cell carcinoma of head and neck. Oral Oncology, 106, 104689.
He, M., Edwards, B. M., Kastelic, D., & Taussig, M. J. (2012). Eukaryotic ribosome display with in situ DNA recovery (pp. 75–85). Ribosome Display and Related Technologies.
Yarian, F., Kazemi, B., & Bandehpour, M. (2018). Identification and characterization of a novel single-chain variable fragment (scFv) antibody against Neisseria meningitidis factor H-binding protein (fHbp). Journal of Medical Microbiology, 67(6), 820–827.
Ahangarzadeh, S., Bandehpour, M., & Kazemi, B. (2017). Selection of single-chain variable fragments specific for Mycobacterium tuberculosis ESAT-6 antigen using ribosome display. Iranian Journal of Basic Medical Sciences, 20(3), 327.
Salimi, F., Moghadam, M. F., & Rajabibazl, M. (2018). Development of a novel anti-HER2 scFv by ribosome display and in silico evaluation of its 3D structure and interaction with HER2, alone and after fusion to LAMP2B. Molecular Biology Reports, 45(6), 2247–2256.
Rothe, A., Nathanielsz, A., Oberhäuser, F., von Strandmann, E. P., Engert, A., & Hudson, P. J. (2008). Ribosome display and selection of human anti-cD22 scFvs derived from an acute lymphocytic leukemia patient. Biological Chemistry, 389(4), 433–439.
Langer, I. (2021). Maturation of scFv 80r for the neutralization of Sars-CoV-2 Rbd using Ribosome Display. Tufts University-Graduate School of Biomedical Sciences.
He, M., & Khan, F. (2005). Ribosome display: Next-generation display technologies for production of antibodies in vitro. Expert Review of Proteomics, 2(3), 421–430.
Rudnick, S. I., & Adams, G. P. (2009). Affinity and avidity in antibody-based tumor targeting. Cancer Biotherapy and Radiopharmaceuticals, 24(2), 155–161.
Sela-Culang, I., Kunik, V., & Ofran, Y. (2013). The structural basis of antibody-antigen recognition. Frontiers in Immunology, 4, 302.
Birtalan, S., Zhang, Y., Fellouse, F. A., Shao, L., Schaefer, G., & Sidhu, S. S. (2008). The intrinsic contributions of tyrosine, serine, glycine and arginine to the affinity and specificity of antibodies. Journal of Molecular Biology, 377(5), 1518–1528.
Bandehpour, M., Ahangarzadeh, S., Yarian, F., Lari, A., & Farnia, P. (2017). In silico evaluation of the interactions among two selected single chain variable fragments (scFvs) and ESAT-6 antigen of Mycobacterium tuberculosis. Journal of Theoretical and Computational Chemistry, 16(08), 1750069.
Bandehpour, M., Yarian, F., & Ahangarzadeh, S. (2017). Bioinformatics evaluation of novel ribosome display-selected single chain variable fragment (scFv) structure with factor H binding protein through docking. Journal of Theoretical and Computational Chemistry, 16(03), 1750021.
Funding
This research is supported by the Iran National Science Foundation (INSF) (Grant No. 98000339) as a flowship for PhD dissertation of Sepideh Ghani and this article is extracted from Dr. Ghani’s PhD thesis.
Author information
Authors and Affiliations
Contributions
SG wrote original draft and performed the experiment and data analysis. MB supervised the study and provided the funding and project administration. FY, KB, and BK conducted guidance and review and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All animal experiments were conducted in accordance with the guideline approved by the ethics committee of Shahid Beheshti University of Medical Sciences (Ethics code: IR.SBMU.REC.1397.129).
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 27.7 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghani, S., Bandehpour, M., Yarian, F. et al. Production of a Ribosome-Displayed Mouse scFv Antibody Against CD133, Analysis of Its Molecular Docking, and Molecular Dynamic Simulations of Their Interactions. Appl Biochem Biotechnol 196, 1399–1418 (2024). https://doi.org/10.1007/s12010-023-04609-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04609-4