Abstract
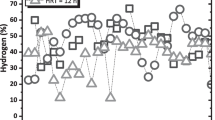
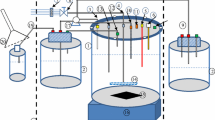
The continuous multiple tube reactor (CMTR) has been developed as a promising technology to maximize biohydrogen production (BHP) by dark fermentation (DF) by preventing excess biomass accumulation, leading to suboptimum values of specific organic loading rates (SOLR). However, previous experiences failed to achieve stable and continuous BHP in this reactor, as the low biomass retention capacity in the tube region limited controlling the SOLR. This study goes beyond the evaluation of the CMTR for DF by inserting grooves in the inner wall of the tubes to ensure better cell attachment. The CMTR was monitored in 4 assays at 25ºC using sucrose-based synthetic effluent. The hydraulic retention time (HRT) was fixed at 2 h, while the COD varied between 2–8 g L−1 to obtain organic loading rates in the 24 – 96 g COD L−1 d−1. Long-term (90 d) BHP was successfully attained in all conditions due to the improved biomass retention capacity. Optimal values for the SOLR (4.9 g COD g−1 VSS d−1) were observed when applying up to 48 g COD L−1 d−1, in which BHP was maximized. These patterns indicate a favorable balance between biomass retention and washout was naturally achieved. The CMTR looks promising for continuous BHP and is exempt from additional biomass discharge strategies.




Similar content being viewed by others
References
Fuess, L. T., Kiyuna, L. S. M., Ferraz Júnior, A. D. N., Persinoti, G. F., Squina, F. M., Garcia, M. L., & Zaiat, M. (2017). Thermophilic two-phase anaerobic digestion using an innovative fixed-bed reactor for enhanced organic matter removal and bioenergy recovery from sugarcane vinasse. Applied Energy, 189, 480–491. https://doi.org/10.1016/j.apenergy.2016.12.071
Lucas, S. D. M., Peixoto, G., Mockaitis, G., Zaiat, M., & Gomes, S. D. (2015). Energy recovery from agro-industrial wastewaters through biohydrogen production: Kinetic evaluation and technological feasibility. Renewable Energy, 75, 496–504. https://doi.org/10.1016/j.renene.2014.10.025
Hallenbeck, P. C., Abo-Hashesh, M., & Ghosh, D. (2012). Strategies for improving biological hydrogen production. Bioresource Technology, 110, 1–9. https://doi.org/10.1016/j.biortech.2012.01.103
Preethi, Usman, T. M. M., Banu, J. R., Gunasekaran, M., & Kumar, G. (2019). Biohydrogen production from industrial wastewater: An overview. Bioresource Technology Reports, 7, 100287. https://doi.org/10.1016/j.biteb.2019.100287
Fernandes, B. S., Peixoto, G., Albrecht, F. R., Aguila, N. K. S., & Zaiat, M. (2010). Potential to produce biohydrogen from various wastewaters. Energy for Sustainable Development, 14, 143–148. https://doi.org/10.1016/j.esd.2010.03.004
Anzola-Rojas, M. P., Zaiat, M., & De Wever, H. (2016). Improvement of hydrogen production via ethanol-type fermentation in an anaerobic down-flow structured bed reactor. Bioresource Technology, 202, 42–49. https://doi.org/10.1016/j.biortech.2015.11.084
Blanco, V. M. C., Fuess, L. T., & Zaiat, M. (2017). Calcium dosing for the simultaneous control of biomass retention and the enhancement of fermentative biohydrogen production in an innovative fixed-film bioreactor. International Journal of Hydrogen Energy, 42, 12181–12196. https://doi.org/10.1016/j.ijhydene.2017.02.180
Chen, W. M., Tseng, Z. J., Lee, K. S., & Chang, J. S. (2005). Fermentative hydrogen production with Clostridium butyricum CGS5 isolated from anaerobic sewage sludge. International Journal of Hydrogen Energy, 30, 1063–1070. https://doi.org/10.1016/j.ijhydene.2004.09.008
Ginkel, S. V., Sung, S., & Lay, J. J. (2001). Biohydrogen production as a function of pH and substrate concentration. Environmental Science and Technology, 35, 4726–4730. https://doi.org/10.1021/es001979r
Elbeshbishy, E., Dhar, B. R., Nakhla, G., & Lee, H. S. (2017). A critical review on inhibition of dark biohydrogen fermentation. Renewable and Sustainable Energy Reviews, 79, 656–668. https://doi.org/10.1016/j.rser.2017.05.075
Castelló, E., Braga, L., Fuentes, L., & Etchebehere, C. (2018). Possible causes for the instability in the H2 production from cheese whey in a CSTR. International Journal of Hydrogen Energy, 43, 2654–2665. https://doi.org/10.1016/j.ijhydene.2017.12.104
Ferraz Júnior, A. D. N., Etchebehere, C., & Zaiat, M. (2015). High organic loading rate on thermophilic hydrogen production and metagenomic study at an anaerobic packed-bed reactor treating a residual liquid stream of a Brazilian biorefinery. Bioresource Technology, 186, 81–88. https://doi.org/10.1016/j.biortech.2015.03.035
Fuess, L. T., Zaiat, M., & Nascimento, C. A. O. (2019). Novel insights on the versatility of biohydrogen production from sugarcane vinasse via thermophilic dark fermentation: Impacts of pH-driven operating strategies on acidogenesis metabolite profiles. Bioresource Technology, 286, 121379. https://doi.org/10.1016/j.biortech.2019.121379
Gomes, S. D., Fuess, L. T., Penteado, E. D., Lucas, S. D. M., Gotardo, J. T., & Zaiat, M. (2015). The application of an innovative continuous multiple tube reactor as a strategy to control the specific organic loading rate for biohydrogen production by dark fermentation. Bioresource Technology, 197, 201–207. https://doi.org/10.1016/j.biortech.2015.08.077
Fuess, L. T., Kiyuna, L. S. M., Garcia, M. L., & Zaiat, M. (2016). Operational strategies for long-term biohydrogen production from sugarcane stillage in a continuous acidogenic packed-bed reactor. International Journal of Hydrogen Energy, 41, 8132–8145. https://doi.org/10.1016/j.ijhydene.2015.10.143
Anzola-Rojas, M. P., & Zaiat, M. (2016). A novel anaerobic down-flow structured-bed reactor for long-term stable H2 energy production from wastewater. Journal of Chemical Technology and Biotechnology, 91, 1551–1561. https://doi.org/10.1002/jctb.4754
Castelló, E., Ferraz-Junior, A. D. N., Andreani, C., Anzola-Rojas, M. P., Borzacconi, L., Buitrón, G., Carrillo-Reyes, J., Gomes, S. D., Maintinguer, S. I., Moreno-Andrade, I., Palomo-Briones, R., Razo-Flores, E., Schiappacasse-Dasati, M., Tapia-Venegas, E., Valdez-Vázquez, I., Vesga-Baron, A., Zaiat, M., & Etchebehere, C. (2020). Stability problems in the hydrogen production by dark fermentation: Possible causes and solutions. Renewable and Sustainable Energy Reviews, 119, 109602. https://doi.org/10.1016/j.rser.2019.109602
Babu, V. L., Mohan, S. V., & Sarma, P. N. (2009). Influence of reactor configuration on fermentative hydrogen production during wastewater treatment. International Journal of Hydrogen Energy, 34, 3305–3312. https://doi.org/10.1016/j.ijhydene.2009.02.011
Arimi, M. M., Knodel, J., Kiprop, A., Namango, S. S., Zhang, Y., & Geißen, S. U. (2015). Strategies for improvement of biohydrogen production from organic-rich wastewater: A review. Biomass and Bioenergy, 75, 101–118. https://doi.org/10.1016/j.biombioe.2015.02.011
Hafez, H., Nakhla, G., El Naggar, M. H., Elbeshbishy, E., & Baghchehsaraee, B. (2010). Effect of organic loading on a novel hydrogen bioreactor. International Journal of Hydrogen Energy, 35, 81–92. https://doi.org/10.1016/j.ijhydene.2009.10.051
Anzola-Rojas, M. P., Fonseca, S. G., Silva, C. C., Oliveira, V. M., & Zaiat, M. (2015). The use of the carbon/nitrogen ratio and specific organic loading rate as tools for improving biohydrogen production in fixed-bed reactors. Biotechnol. Reports, 5, 46–54. https://doi.org/10.1016/j.btre.2014.10.010
Muri, P., Marinšek-Logar, R., Djinović, P., & Pintar, A. (2018). Influence of support materials on continuous hydrogen production in anaerobic packed-bed reactor with immobilized hydrogen producing bacteria at acidic conditions. Enyzme and Microbial Technology, 111, 87–96. https://doi.org/10.1016/j.enzmictec.2017.10.008
Braga, A. F. M., Ferraz Júnior, A. D. N., & Zaiat, M. (2016). Thermophilic biohydrogen production using a UASB reactor: Performance during long-term operation. Journal of Chemical Technology and Biotechnology, 91, 967–976. https://doi.org/10.1002/jctb.4665
Lima, D. M. F., & Zaiat, M. (2012). The influence of the degree of back-mixing on hydrogen production in an anaerobic fixed-bed reactor. International Journal of Hydrogen Energy, 37, 9630–9635. https://doi.org/10.1016/j.ijhydene.2012.03.097
Penteado, E. D., Lazaro, C. Z., Sakamoto, I. K., & Zaiat, M. (2013). Influence of seed sludge and pretreatment method on hydrogen production in packed-bed anaerobic reactors. International Journal of Hydrogen Energy, 38, 6137–6145. https://doi.org/10.1016/j.ijhydene.2013.01.067
Leite, J. A. C., Fernandes, B. S., Pozzi, E., Barboza, M., & Zaiat, M. (2008). Application of an anaerobic packed-bed bioreactor for the production of hydrogen and organic acids. International Journal of Hydrogen Energy, 33, 579–586. https://doi.org/10.1016/j.ijhydene.2007.10.009
APHA. American Public Health Association; AWWA. American Water Works Association; WEF. Water Environment Federation. (2012) Standard methods for the examination of water and wastewater 22. ed. Washington: APHA/AWWA/WEF
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356. https://doi.org/10.1021/ac60111a017
Perna, V., Castelló, E., Wenzel, J., Zampol, C., Lima, D. M. F., Borzacconi, L., Varesche, M. B., Zaiat, M., & Etchebehere, C. (2013). Hydrogen production in an upflow anaerobic packed bed reactor used to treat cheese whey. International Journal of Hydrogen Energy, 38, 54–62. https://doi.org/10.1016/j.ijhydene.2012.10.022
Ferraz Júnior, A. D. N., Koyama, M. H., Araújo Júnior, M. M., & Zaiat, M. (2016). Thermophilic anaerobic digestion of raw sugarcane vinasse. Renewable Energy, 89, 245–252. https://doi.org/10.1016/j.renene.2015.11.064
Gavala, H. N., Skiadas, I. V., & Ahring, B. K. (2006). Biological hydrogen production in suspended and attached growth anaerobic reactor systems. International Journal of Hydrogen Energy, 31, 1164–1175. https://doi.org/10.1016/j.ijhydene.2005.09.009
Lo, Y. C., Chen, W. M., Hung, C. H., Chen, S. D., & Chang, J. S. (2008). Dark H2 fermentation from sucrose and xylose using H2-producing indigenous bacteria: Feasibility and kinetic studies. Water Research, 42, 827–842. https://doi.org/10.1016/j.watres.2007.08.023
Inoue, R. K., Lima, D. M. F., Rodrigues, J. A. D., Ratusznei, S. M., & Zaiat, M. (2014). Effect of organic loading rate and fill time on the biohydrogen production in a mechanically stirred AnSBBR treating synthetic sucrose-sased wastewater. Applied Biochemistry and Biotechnology, 174, 2326–2349. https://doi.org/10.1007/s12010-014-1205-7
Kyazze, G., Martinez-Perez, N., Dinsdale, R., Premier, G. C., Hawkes, F. R., Guwy, A. J., & Hawkes, D. L. (2006). Influence of substrate concentration on the stability and yield of continuous biohydrogen production. Biotechnology and Bioengineering, 93, 971–979. https://doi.org/10.1002/bit.20802
Gomes, S. D., Fuess, L. T., Mañunga, T., Gomes, P. C. F. L., & Zaiat, M. (2016). Bacteriocins of lactic acid bacteria as a hindering factor for biohydrogen production from cassava flour wastewater in a continuous multiple tube reactor. International Journal of Hydrogen Energy, 41, 8120–8131. https://doi.org/10.1016/j.ijhydene.2015.11.186
Manssouri, M., Rodrigues, J. A. D., Ratusznei, S. M., & Zaiat, M. (2013). Effects of organic loading, influent concentration, and feed time on biohydrogen production in a mechanically stirred AnSBBR treating sucrose-based wastewater. Applied Biochemistry and Biotechnology, 171, 1832–1854. https://doi.org/10.1007/s12010-013-0457-y
Andreani, C. L., Tonello, T. U., Mari, A. G., Leite, L. C. C., Campaña, H. D., Lopes, D. D., Rodrigues, J. A. D., & Gomes, S. D. (2019). Impact of operational conditions on development of the hydrogen-producing microbial consortium in an AnSBBR from cassava wastewater rich in lactic acid. International Journal of Hydrogen Energy, 44, 1474–1482. https://doi.org/10.1016/j.ijhydene.2018.11.155
Corbari, S. D. M. L., Andreani, C. L., Torres, D. G. B., Eng, F., & Gomes, S. D. (2019). Strategies to improve the biohydrogen production from cassava wastewater in fixed-bed reactors. International Journal of Hydrogen Energy, 44, 17214–17223. https://doi.org/10.1016/j.ijhydene.2019.04.242
Gorgeç, F. K., & Karapinar, I. (2019). Production of biohydrogen from waste wheat in continuously operated UPBR: The effect of influent substrate concentration. International Journal of Hydrogen Energy, 44, 17323–17333. https://doi.org/10.1016/j.ijhydene.2018.12.213
Reis, C., & Silva, E. L. (2011). Effect of upflow velocity and hydraulic retention time in anaerobic fluidized-bed reactors used for hydrogen production. Chemical Engineering Journal, 172, 28–36.
Fernandes, B. S., Saavedra, N. K., Maintinguer, S. I., Sette, L. D., Oliveira, V. M., Varesche, M. B. A., & Zaiat, M. (2013). The effect of biomass immobilization support material and bed porosity on hydrogen production in an upflow anaerobic packed-bed bioreactor. Applied Biochemistry and Biotechnology, 170, 1348–1366. https://doi.org/10.1007/s12010-013-0262-7
Arantes, M. K., Alves, H. J., Sequinel, R., & Silva, E. A. (2017). Treatment of brewery wastewater and its use for biological production of methane and hydrogen. International Journal of Hydrogen Energy, 42, 26243–26256. https://doi.org/10.1016/j.ijhydene.2017.08.206
Guo, X. M., Trably, E., Latrille, E., Carrère, H., & Steyer, J. P. (2010). Hydrogen production from agricultural waste by dark fermentation: A review. International Journal of Hydrogen Energy, 35, 10660–10673. https://doi.org/10.1016/j.ijhydene.2010.03.008
Matsumoto, M., & Nishimura, Y. (2007). Hydrogen production by fermentation using acetic acid and lactic acid. Journal of Bioscience and Bioengineering, 103, 236–241. https://doi.org/10.1263/jbb.103.236
Madigan, M.T., Martinko, J.M., Bender, K.S., Buckley, D.H., Stahl, D.A. (2014). Brock biology of microorganisms, fourteenth ed. Pearson, USA. ISBN-13 978-0321897398.
Kawano, T., Wada, K., Li, Y. Y., & Noike, T. (2004). Effects of substrate concentration and pH on hydrogen fermentation of mixed substrate by microflora. Journal of Japan Society on Water Environment, 27, 473–479. https://doi.org/10.2965/jswe.27.473
Etchebehere, C., Castelló, E., Wenzel, J., Anzola-Rojas, M. P., Borzacconi, L., Buitrón, G., Cabrol, L., Carminato, V. M., Carrillo-Reyes, J., Cisneros-Pérez, C., Fuentes, L., Moreno-Andrade, I., Razo-Flores, E., Filippi, G. R., Tapia-Venegas, E., Toledo-Alarcón, J., & Zaiat, M. (2016). Microbial communities from 20 different hydrogen-producing reactors studied by 454 pyrosequencing. Applied Microbiology and Biotechnology, 100, 3371–3384. https://doi.org/10.1007/s00253-016-7325-y
Yang, P., Zhang, R., McGarvey, J. A., & Benemann, J. R. (2007). Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. International Journal of Hydrogen Energy, 32, 4761–4771. https://doi.org/10.1016/j.ijhydene.2007.07.038
Estevam, A., Arantes, M. K., Andrigheto, C., Fiorini, A., da Silva, E. A., & Alves, H. J. (2018). Production of biohydrogen from brewery wastewater using Klebsiella pneumoniae isolated from the environment. International Journal of Hydrogen Energy, 43, 4276–4283. https://doi.org/10.1016/j.ijhydene.2018.01.052
Chen, C. C., Lin, C. Y., & Chang, J. S. (2001). Kinetics of hydrogen production with continuous anaerobic cultures utilizing sucrose as the limiting substrate. Applied Microbiology and Biotechnology, 57, 56–64. https://doi.org/10.1007/s002530100747
Hafez, H., Baghchehsaraee, B., Nakhla, G., Karamanev, D., Margaritis, A., & El Naggar, H. (2009). Comparative assessment of decoupling of biomass and hydraulic retention times in hydrogen production bioreactors. International Journal of Hydrogen Energy, 34, 7603–7611. https://doi.org/10.1016/j.ijhydene.2009.07.060
Funding
The authors acknowledge the financial support of Araucaria Foundation (grant number 20/2015) and the National Council for Scientific and Technological Development (CNPq, grant number 311741/2018–5).
Author information
Authors and Affiliations
Contributions
Ana Paula Trevisan: Conceptualization, methodology, writing, experiments. Simone Gomes: resources, writing and supervision. Marcelo Zaiat: review. Lucas Fuess: review. Willian de Souza: data analysis and writing. Eduardo Lied: writing – review and editing. Benedito Gomes: resources.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The grooves inside the tubes in a worm-like fashion were adequate for the biomass attachment.
• CMTR maintained continuous H2 production in long-term operation (90 d) and the ability to recover from performance losses.
• CMTR operated steadily at specific organic loading rate values regardless of the ideal range.
• The larger values of production of H2 were associated with the best conditions of biomass adhesion on the CMTR tube walls.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trevisan, A.P., Lied, E.B., Fuess, L.T. et al. Improving the Continuous Multiple Tube Reactor: an Innovative Bioreactor Configuration with Great Potential for Dark Fermentation Processes. Appl Biochem Biotechnol 196, 457–477 (2024). https://doi.org/10.1007/s12010-023-04553-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04553-3