Abstract
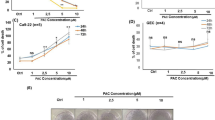
Until now, chemotherapy, which has a series of side effects, has been the most widely employed treatment for different types of cancer. However, bioactive products have been utilized as alternative medicines for tumors due to their bioactivities with low or no side effects in normal cells. This research reported for the first time that curcumin (CUR) and paclitaxel (PTX) have significant anti-cancer activity against normal human gingival fibroblast (HGF) and tongue squamous cell carcinoma fibroblast (TSCCF) cell lines. The results showed that CUR (13.85 µg mL−1) and PTX (8.17 µg mL−1) significantly inhibited TSCCF cell viability, with no significant effect on normal HGF cells. SEM showed morphological changes in cells treated with CUR and PTX, especially with TSCCF cells, compared to HGF normal cells. For TSCCF, the results showed the highest necrosis was achieved with CUR (58.8%) and PTX (39%) as compared to the control (2.99%). For normal HGF cells, the highest early and late apoptosis was achieved with PTX. Further, DCFH-DA analyses showed no significant ROS stimulation in TSCCF and HGF cell lines treated with CUR and PTX. The 1H NMR analysis results show the presence of methoxy and hydroxyl groups and aromatic hydrogens in the CUR structure. In conclusion, the results confirmed that CUR is more specific to the oral cancer cells but not normal cells by inducing apoptosis in a dose- and time-dependent manner, with decreased TSCCF cell viability, and the cytotoxicity of CUR and PTX is not through the ROS pathway.






Similar content being viewed by others
Data Availability
The published article includes all data generated or analyzed during this study. The study’s raw data are available from the corresponding author upon reasonable request.
References
Tomeh, M. A., Hadianamrei, R., & Zhao, X. (2019). A review of curcumin and its derivatives as anticancer agents. International Journal of Molecular Sciences, 20(5), 1033.
Zhang, Q., Alyami, N. M., Alyami, H. M., Natarajan, N., & Elayappan, P. K. (2022). Influence of Padina gymnospora on apoptotic proteins of oral cancer cells—a proteome-wide analysis. Applied Biochemistry and Biotechnology, 194(12), 5945–5962.
Zhang, Y., Liu, P., Su, W., Aodeng, G., & Zhao, H. (2022). Fibroblast growth factor 3 Is associated with tongue squamous cell carcinoma: a controlled study. Computational and Mathematical Methods in Medicine, 2022.
Li, H., Zhang, J., Chen, S. W., Liu, L. L., Li, L., Gao, F., Zhuang, S. M., Wang, L. P., Li, Y., & Song, M. (2015). Cancer-associated fibroblasts provide a suitable microenvironment for tumor development and progression in oral tongue squamous cancer. Journal of Translational Medicine, 13(1), 1–10.
Alanazi, H., Park, H. J., Chakir, J., Semlali, A., & Rouabhia, M. (2018). Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food and Chemical Toxicology, 118, 390–398.
Nawara, H. M., Afify, S. M., Hassan, G., Zahra, M. H., Seno, A., & Seno, M. (2021). Paclitaxel-based chemotherapy targeting cancer stem cells from mono-to combination therapy. Biomedicines, 9(5), 500.
Liu, Z., Huang, P., Law, S., Tian, H., Leung, W., & Xu, C. (2018). Preventive effect of curcumin against chemotherapy-induced side-effects. Frontiers in Pharmacology, 9, 1374.
Gao, X., Wang, B., Wu, Q., Wei, X., Zheng, F., Men, K., Shi, H., Huang, N., Wei, Y., & Gong, C. (2015). Combined delivery and anti-cancer activity of paclitaxel and curcumin using polymeric micelles. Journal of Biomedical Nanotechnology, 11(4), 578–589.
Jiang, D., Rasul, A., Batool, R., Sarfraz, I., Hussain, G., Mateen Tahir, M., Qin, T., Selamoglu, Z., Ali, M., Li, J., & Li, X. (2019). Potential anticancer properties and mechanisms of action of formononetin. BioMed Research International, 2019.
Wu, J., Cai, Z., Wei, X., Chen, M., Ying, S., Shi, L., Xu, R.A., He, F., Liang, G., & Zhang, X. (2015). Anti-lung cancer activity of the curcumin analog JZ534 in vitro. BioMed Research International, 2015.
Rodrigues, F. C., Kumar, N. A., & Thakur, G. (2019). Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. European Journal of Medicinal Chemistry, 177, 76–104.
Liu, H. T., & Ho, Y. S. (2018). Anticancer effect of curcumin on breast cancer and stem cells. Food Science and Human Wellness, 7(2), 134–137.
Koohpar, Z. K., Entezari, M., Movafagh, A., & Hashemi, M. (2015). Anticancer activity of curcumin on human breast adenocarcinoma: Role of Mcl-1 gene. Iranian Journal of Cancer Prevention, 8(3), e2331.
Hussein, H. A., Syamsumir, D. F., Radzi, S. A. M., Siong, J. Y. F., Zin, N. A. M., & Abdullah, M. A. (2020). Phytochemical screening, metabolite profiling and enhanced antimicrobial activities of microalgal crude extracts in co-application with silver nanoparticle. Bioresources and Bioprocessing, 7(1), 1–17.
Hussein, H. A., Maulidiani, M., & Abdullah, M. A. (2020). Microalgal metabolites as anti-cancer/antioxidant agents reduce cytotoxicity of elevated silver nanoparticle levels against non-cancerous vero cells. Heliyon, 6(10), e05263.
Hussein, H. A., Mohamad, H., Ghazaly, M. M., Laith, A. A., & Abdullah, M. A. (2020). Anticancer and antioxidant activities of Nannochloropsis Oculata and Chlorella sp. extracts in co-application with silver nanoparticle. Journal of King Saud University-Science, 32(8), 3486–3494.
Calaf, G. M., Ponce-Cusi, R., & Carrión, F. (2018). Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncology Reports, 40(4), 2381–2388.
Hussein, H. A., Mohamad, H., Ghazaly, M. M., Laith, A. A., & Abdullah, M. A. (2020). Cytotoxic effects of Tetraselmis suecica chloroform extracts with silver nanoparticle co-application on MCF-7, 4 T1, and Vero cell lines. Journal of Applied Phycology, 32(1), 127–143.
Tajudin, T. J. S. A., Mat, N., Siti-Aishah, A. B., Yusran, A. A. M., Alwi, A., & Ali, A. M. (2012). Cytotoxicity, antiproliferative effects, and apoptosis induction of methanolic extract of Cynometra cauliflora Linn. whole fruit on human promyelocytic leukemia HL-60 cells. Evidence-Based Complementary and Alternative Medicine, 2012, Article ID 127373.
Tan, X., Sidell, N., Mancini, A., Huang, R. P., Wang, S., Horowitz, I. R., Liotta, D. C., Taylor, R. N., & Wieser, F. (2010). Multiple anticancer activities of EF24, a novel curcumin analog, on human ovarian carcinoma cells. Reproductive Sciences, 17(10), 931–940.
Kim, H., & Xue, X. (2020). Detection of total reactive oxygen species in adherent cells by 2’, 7’-Dichlorodihydrofluorescein diacetate staining. Journal of Visualized Experiments, 160, e60682.
Allegra, A., Innao, V., Russo, S., Gerace, D., Alonci, A., & Musolino, C. (2017). Anticancer activity of curcumin and its analogues: Preclinical and clinical studies. Cancer Investigation, 35(1), 1–22.
El-Tabba, R. M., Mathew, P., Masocha, W., & Khajah, M. A. (2019). COL-3 enhances the anti-proliferative and pro-apoptotic effects of paclitaxel in breast cancer cells. Oncology Reports, 41(1), 630–642.
Kang, J., Chen, J., Shi, Y., Jia, J., & Zhang, Y. (2005). Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochemical Pharmacology, 69(8), 1205–1213.
NavaneethaKrishnan, S., Rosales, J. L., & Lee, K. Y. (2019). ROS-mediated cancer cell killing through dietary phytochemicals. Oxidative Medicine and Cellular Longevity, 2019.
Meiyanto, E., Putri, D. D. P., Susidarti, R. A., Murwanti, R., Sardjiman, S., Fitriasari, A., Husnaa, U., Purnomo, H., & Kawaichi, M. (2014). Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-kB activation. Asian Pacific Journal of Cancer Prevention, 15(1), 179–184.
Agrawal, N., & Jaiswal, M. (2022). Bioavailability enhancement of curcumin via esterification processes: a review. European Journal of Medicinal Chemistry Reports, 100081
Mansouri, K., Rasoulpoor, S., Daneshkhah, A., Abolfathi, S., Salari, N., Mohammadi, M., Rasoulpoor, S., & Shabani, S. (2020). Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer, 20(1), 1–11.
Chakravarti, N., Kadara, H., Yoon, D. J., Shay, J. W., Myers, J. N., Lotan, D., Sonenberg, N., & Lotan, R. (2010). Differential inhibition of protein translation machinery by curcumin in normal, immortalized, and malignant oral epithelial cells. Cancer Prevention Research, 3(3), 331–338.
Ramachandran, C., & You, W. (1999). Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Research and Treatment, 54(3), 269–278.
Xi, Y., Gao, H., Callaghan, M. U., Fribley, A. M., Garshott, D. M., Xu, Z. X., Zeng, Q., & Li, Y. L. (2015). Induction of BCL2-interacting killer, BIK, is mediated for anti-cancer activity of curcumin in human head and neck squamous cell carcinoma cells. Journal of Cancer, 6(4), 327–332.
Bolat, Z. B., Islek, Z., Demir, B. N., Yilmaz, E. N., Sahin, F., & Ucisik, M. H. (2020). Curcumin-and piperine-loaded emulsomes as combinational treatment approach enhance the anticancer activity of curcumin on HCT116 colorectal cancer model. Frontiers in Bioengineering and Biotechnology, 8, 50.
Acedo, P., Stockert, J. C., Cañete, M., & Villanueva, A. (2014). Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death & Disease, 5(3), e1122–e1122.
Montón, H., Parolo, C., Aranda-Ramos, A., Merkoçi, A., & Nogués, C. (2015). Annexin-V/quantum dot probes for multimodal apoptosis monitoring in living cells: Improving bioanalysis using electrochemistry. Nanoscale, 7(9), 4097–4104.
Kwan, Y. P., Saito, T., Ibrahim, D., Al-Hassan, F. M. S., Ein Oon, C., Chen, Y., Jothy, S. L., Kanwar, J. R., & Sasidharan, S. (2016). Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharmaceutical Biology, 54(7), 1223–1236.
Perrone, D., Ardito, F., Giannatempo, G., Dioguardi, M., Troiano, G., Lo Russo, L., De Lillo, A., Laino, L., & Lo Muzio, L. (2015). Biological and therapeutic activities, and anticancer properties of curcumin. Experimental and Therapeutic Medicine, 10(5), 1615–1623.
Al-Asmari, A. K., Riyasdeen, A., & Islam, M. (2018). Scorpion venom causes apoptosis by increasing reactive oxygen species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell lines. Journal of Evidence-Based Integrative Medicine, 23, 2156587217751796.
Redza-Dutordoir, M., & Averill-Bates, D. A. (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta (BBA)- Molecular Cell Research, 1863(12), 2977–2992.
Jang, D. K., Lee, I. S., Shin, H. S., & Yoo, H. M. (2020). 2α-Hydroxyeudesma-4, 11 (13)-Dien-8β, 12-Olide isolated from inula britannica induces apoptosis in diffuse large B-cell lymphoma cells. Biomolecules, 10(2), 324.
Hanif, N., Hermawan, A., & Meiyanto, E. (2019). Caesalpinia sappan L. ethanolic extract decrease intracellular ros level and senescence of 4t1 breast cancer cells. Indonesian Journal of Cancer Chemoprevention, 10(1), 16–23.
Shishodia, S. (2013). Molecular mechanisms of curcumin action: Gene expression. BioFactors, 39(1), 37–55.
Zhou, M., Fan, C., & Tian, N. (2015). Effects of curcumin on the gene expression profile of L-02 cells. Biomedical Reports, 3(4), 519–526.
Rao, J., Xu, D. R., Zheng, F. M., Long, Z. J., Huang, S. S., Wu, X., Zhou, W. H., Huang, R. W., & Liu, Q. (2011). Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. Journal of Translational Medicine, 9, 1–15.
Banerjee, S., Singh, S. K., Chowdhury, I., Lillard, J. W., Jr., & Singh, R. (2017). Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Frontiers in Bioscience (Elite edition), 9, 235.
Li, N., Wen, S., Chen, G., & Wang, S. (2020). Antiproliferative potential of piperine and curcumin in drug-resistant human leukemia cancer cells are mediated via autophagy and apoptosis induction, S-phase cell cycle arrest and inhibition of cell invasion and migration. Journal of B.U.ON., 25, 401–406.
Scott, D. W., & Loo, G. (2004). Curcumin-induced GADD153 gene up-regulation in human colon cancer cells. Carcinogenesis, 25(11), 2155–2164.
Narayan, S. (2004). Curcumin, a multi-functional chemopreventive agent, blocks growth of colon cancer cells by targeting β-catenin-mediated transactivation and cell–cell adhesion pathways. Journal of Molecular Histology, 35, 301–307.
Fetoni, A. R., Eramo, S. L., Paciello, F., Rolesi, R., Podda, M. V., Troiani, D., & Paludetti, G. (2014). Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otology & Neurotology, 35(5), e169–e177.
Flores-Frias, E. A., Barba, V., Lucio-Garcia, M. A., Lopez-Cecenes, R., Porcayo-Calderon, J., & Gonzalez-Rodriguez, J. G. (2019). Use of Curcuma and curcumin as a green corrosion inhibitors for carbon steel in sulfuric acid. International Journal of Electrochemical Science, 14, 5026–5041.
Ahmed, M., Qadir, M. A., Shafiq, M. I., Muddassar, M., Hameed, A., Arshad, M. N., & Asiri, A. M. (2017). Curcumin: Synthesis optimization and in silico interaction with cyclin dependent kinase. Acta Pharmaceutica, 67(3), 385–395.
Manju, S., & Sreenivasan, K. (2011). Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. Journal of Colloid and Interface Science, 359(1), 318–325.
Valand, N. N., Patel, M. B., & Menon, S. K. (2015). Curcumin-p-sulfonatocalix [4] resorcinarene (p-SC [4] R) interaction: Thermo-physico chemistry, stability and biological evaluation. RSC Advances, 5(12), 8739–8752.
Singh, A. K., Yadav, S., Sharma, K., Firdaus, Z., Aditi, P., Neogi, K., Bansal, M., Gupta, M. K., Shanker, A., Singh, R. K., & Prakash, P. (2018). Quantum curcumin mediated inhibition of gingipains and mixed-biofilm of Porphyromonas gingivalis causing chronic periodontitis. RSC Advances, 8(70), 40426–40445.
Acknowledgements
The authors thank Dr. Benyamin and the science officers at the University of Tehran for assisting with the facilities for the experiments.
Author information
Authors and Affiliations
Contributions
Hanaa Ali Hussein: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; wrote the paper.
Fatin L. Khaphi: conceived and designed the experiments; contributed reagents, materials, analysis tools, or data; analyzed and interpreted the data; wrote the paper.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Inapplicable.
Patient Consent for Publication
Inapplicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hussein, H.A., Khaphi, F.L. The Apoptotic Activity of Curcumin Against Oral Cancer Cells Without Affecting Normal Cells in Comparison to Paclitaxel Activity. Appl Biochem Biotechnol 195, 5019–5033 (2023). https://doi.org/10.1007/s12010-023-04454-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04454-5