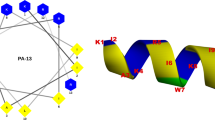
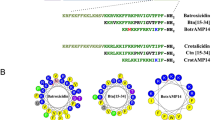
Abstract
Antimicrobial peptides are becoming a new generation of antibiotics due to their therapeutic potential and ability to decrease drug-resistant bacteria development. Cathelicidins are known as effective peptides of vertebrate immunity that play crucial roles in the defensive strategy against pathogens. To improve its potency, the RN15 antibacterial peptide derived from the cathelin domain of Crocodylus siamensis cathelicidin has been modified and its antimicrobial properties investigated. Peptides were derived by template-based and physicochemical designation. The RN15 derivative peptides were predicted through their structure modeling, antimicrobial potency, and peptide-membrane calculation. The antimicrobial and cytotoxic activities of candidate peptides were investigated. Simultaneous consideration of physicochemical characteristics, secondary structure modeling, and the result of antimicrobial peptide tools prediction indicated that RN15m4 peptide was a candidate derivative antimicrobial peptide. The RN15m4 peptide expresses antimicrobial activity against most Gram-positive and Gram-negative bacteria and fungi with a lower minimum inhibition concentration (MIC) than the parent peptide. Besides, the time-killing assay shows that the designed peptide performed its ability to quickly kill bacteria better than the original peptide. Scanning electron microscopy (SEM) displayed the destruction of the bacterial cell membrane caused by the RN15m4 peptide. In addition, the RN15m4 peptide exhibits low hemolytic activity and low cytotoxic activity as good as the template peptide. The RN15m4 peptide performs a range of antimicrobial activities with low cell toxicity. Our study has illustrated the combination approach to peptide design for potent antibiotic peptide discovery.





Similar content being viewed by others
Data Availability
All data are provided in the manuscript.
References
Pontes, D. S., de Araujo, R. S. A., Dantas, N., Scotti, L., Scotti, M. T., de Moura, R. O., & Mendonca-Junior, F. J. B. (2018). Genetic mechanisms of antibiotic resistance and the role of antibiotic adjuvants. Current Topics in Medicinal Chemistry, 18, 42–74.
Teixeira, V., Feio, M. J., & Bastos, M. (2012). Role of lipids in the interaction of antimicrobial peptides with membranes. Progress in Lipid Research, 51, 149–177.
Forde, E., & Devocelle, M. (2015). Pro-moieties of antimicrobial peptide prodrugs. Molecules, 20, 1210–1227.
Porto, W. F., Silva, O. N., & Franco, O. L. (2012). Prediction and rational design of antimicrobial peptides. In Protein Structure, Faraggi, E., Ed., InTech
Avila, E. E. (2017). Functions of antimicrobial peptides in vertebrates. Current Protein and Peptide Science, 18, 1098–1119.
Ramanathan, B., Davis, E. G., Ross, C. R., & Blecha, F. (2002). Cathelicidins: Microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes and Infection, 4, 361–372.
de Latour, F. A., Amer, L. S., Papanstasiou, E. A., Bishop, B. M., & van Hoek, M. L. (2010). Antimicrobial activity of the Naja atra cathelicidin and related small peptides. Biochemical and Biophysical Research Communications, 396, 825–830.
Chen, W., Yang, B., Zhou, H., Sun, L., Dou, J., Qian, H., Huang, W., Mei, Y., & Han, J. (2011). Structure-activity relationships of a snake cathelicidin-related peptide, BF-15. Peptides, 32, 2497–2503.
Tajbakhsh, M., Karimi, A., Tohidpour, A., Abbasi, N., Fallah, F., & Akhavan, M. M. (2018). The antimicrobial potential of a new derivative of cathelicidin from Bungarus fasciatus against methicillin-resistant Staphylococcus aureus. Journal of Microbiology, 56, 128–137.
Xing, M., Ji, M., Hu, J., Zhu, T., Chen, Y., Bai, X., Mwangi, J., Mo, G., Lai, R., & Jin, L. (2020). Snake cathelicidin derived peptide inhibits Zika virus infection. Frontiers in Microbiology, 11, 1871.
Oliveira, N. G. J., Cardoso, M. H., Velikova, N., Giesbers, M., Wells, J. M., Rezende, T. M. B., de Vries, R., & Franco, O. L. (2020). Physicochemical-guided design of cathelicidin-derived peptides generates membrane active variants with therapeutic potential. Scientific Reports, 10, 9127.
Gao, J., Wang, Y., & Yu, H. (2021). Molecular design, pharmacology and toxicology optimization of antimicrobial peptide from Hydrophis cyanocinctus, Hc-CATH. Sheng Wu Gong Cheng Xue Bao, 37, 2534–2542.
Cai, S., Lu, C., Liu, Z., Wang, W., Lu, S., Sun, Z., & Wang, G. (2021). Derivatives of gecko cathelicidin-related antioxidant peptide facilitate skin wound healing. European Journal of Pharmacology, 890, 173649.
Preecharram, S., Daduang, S., Bunyatratchata, W., Araki, T., & Thammasirirak, S. (2008). Antibacterial activity from Siamese crocodile (Crocodylus siamensis) serum. African Journal of Biotechnology, 7, 3121–3128.
Pata, S., Yaraksa, N., Daduang, S., Temsiripong, Y., Svasti, J., Araki, T., & Thammasirirak, S. (2011). Characterization of the novel antibacterial peptide Leucrocin from crocodile (Crocodylus siamensis) white blood cell extracts. Developmental and Comparative Immunology, 35, 545–553.
Kommanee, J., Preecharram, S., Daduang, S., Temsiripong, Y., Dhiravisit, A., Yamada, Y., & Thammasirirak, S. (2012). Antibacterial activity of plasma from crocodile (Crocodylus siamensis) against pathogenic bacteria. Annals of Clinical Microbiology and Antimicrobials, 11, 22.
Srihongthong, S., Pakdeesuwan, A., Daduang, S., Araki, T., Dhiravisit, A., & Thammasirirak, S. (2012). Complete amino acid sequence of globin chains and biological activity of fragmented crocodile hemoglobin (Crocodylus siamensis). The Protein Journal, 31, 466–476.
Yaraksa, N., Anunthawan, T., Theansungnoen, T., Daduang, S., Araki, T., Dhiravisit, A., & Thammasirirak, S. (2014). Design and synthesis of cationic antibacterial peptide based on Leucrocin I sequence, antibacterial peptide from crocodile (Crocodylus siamensis) white blood cell extracts. The Journal of Antibiotics (Tokyo), 67, 205–212.
Theansungnoen, T., Maijaroen, S., Jangpromma, N., Yaraksa, N., Daduang, S., Temsiripong, T., Daduang, J., & Klaynongsruang, S. (2016). Cationic antimicrobial peptides derived from Crocodylus siamensis leukocyte extract, revealing anticancer activity and apoptotic induction on human cervical cancer cells. The Protein Journal, 35, 202–211.
Tankrathok, A., Punpad, A., Kongchaiyapoom, M., Sosiangdi, S., Jangpromma, N., Daduang, S., & Klaynongsruang, S. (2019). Identification of the first Crocodylus siamensis cathelicidin gene and RN15 peptide derived from cathelin domain exhibiting antibacterial activity. Biotechnology and Applied Biochemistry, 66, 142–152.
Wang, G., Li, X., & Wang, Z. (2009). APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Research, 37, 933–937.
Pirtskhalava, M., Amstrong, A. A., Grigolava, M., Chubinidze, M., Alimbarashvili, E., Vishnepolsky, B., Gabrielian, A., Rosenthal, A., Hurt, D. E., & Tartakovsky, M. (2021). DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Research, 49, D288–D297.
Thévenet, P., Shen, Y., Maupetit, J., Guyon, F., Derreumaux, P., & Tufféry, P. (2012). PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Research, 40, W288–W293.
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I., & Lomize, A. L. (2012). OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Research, 40, D370–D376.
Jiang, Z., Vasil, A. I., Hale, J. D., Hancock, R. E., Vasil, M. L., & Hodges, R. S. (2008). Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers, 90, 369–383.
Yang, S. T., Shin, S. Y., Lee, C. W., Kim, Y. C., Hahm, K. S., & Kim, J. I. (2003). Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Letters, 540, 229–233.
Huang, Y., He, L., Li, G., Zhai, N., Jiang, H., & Chen, Y. (2014). Role of helicity of α-helical antimicrobial peptides to improve specificity. Protein & Cell, 5, 631–642.
Mishra, A. K., Choi, J., Moon, E., & Baek, K. H. (2018). Tryptophan-rich and proline-rich antimicrobial peptides. Molecules, 23, 815.
Lee, T. H., Hall, K. N., & Aguilar, M. I. (2016). Antimicrobial peptide structure and mechanism of action: A focus on the role of membrane structure. Current Topics in Medicinal Chemistry, 16, 25–39.
Fillion, M., Valois-Paillard, G., Lorin, A., Noël, M., Voyer, N., & Auger, M. (2015). Membrane interactions of synthetic peptides with antimicrobial potential: Effect of electrostatic interactions and amphiphilicity. Probiotics and Antimicrobial Proteins, 7, 66–74.
Juba, M. L., Porter, D. K., Williams, E. H., Rodriguez, C. A., Barksdale, S. M., & Bishop, B. M. (2015). Helical cationic antimicrobial peptide length and its impact on membrane disruption. Biochimica et Biophysica Acta, 1848, 1081–1091.
Acknowledgements
This research was conducted at the Protein and Proteomics Research Center for Commercial and Industrial Purposes (ProCCI), Khon Kaen University, and Kalasin University.
Funding
This work was financially supported by the Thailand Research Fund (TRF) and the Office of the Higher Education Commission (OHEC) (TRG5880148).
Author information
Authors and Affiliations
Contributions
Nisachon Jangpromma analyzed the data. Monruedee Konkchaiyaphum, Arpaporn Punpad, and Sirinthip Sosiangdi performed the experimental work. Sakda Daduang and Sompong Klaynongsruang supervised the study. Anupong Tankrathok analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
All authors have their consent to participate.
Consent to Publish
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jangpromma, N., Konkchaiyaphum, M., Punpad, A. et al. Rational Design of RN15m4 Cathelin Domain-Based Peptides from Siamese Crocodile Cathelicidin Improves Antimicrobial Activity. Appl Biochem Biotechnol 195, 1096–1108 (2023). https://doi.org/10.1007/s12010-022-04210-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04210-1