Abstract
In the current scenario of the coronavirus pandemic caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), considerable efforts have been made to control the pandemic by the development of a strong immune system through massive vaccination. Just after the discovery of the genetic sequences of SARS-CoV-2, the development of vaccines became the prime focus of scientists around the globe. About 200 SARS-CoV-2 candidate vaccines have already been entered into preclinical and clinical trials. Various traditional and novel approaches are being utilized as a broad range of platforms. Viral vector (replicating and non-replicating), nucleic acid (DNA and RNA), recombinant protein, virus-like particle, peptide, live attenuated virus, an inactivated virus approaches are the prominent attributes of the vaccine development. This review article includes the current knowledge about the platforms used for the development of different vaccines, their working principles, their efficacy, and the impacts of COVID-19 vaccines on thrombosis. We provide a detailed description of the vaccines that are already approved by administrative authorities. Moreover, various strategies utilized in the development of emerging vaccines that are in the trial phases along with their mode of delivery have been discussed along with their effect on thrombosis and gastrointestinal disorders.
Similar content being viewed by others
Introduction
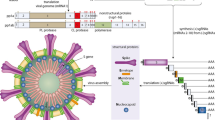
A recently emerged Coronavirus disease 2019 (COVID-19) is associated with a novel virus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). It is a beta-coronavirus that caused high morbidity and fatality throughout the globe [1]. According to the recent annotation (GenBank: NC_045512.2), SARS-CoV-2 is an enveloped virus having single-stranded RNA of ∼30 kb, where conserved structural proteins (membrane protein, spike protein, nucleocapsid protein, envelope protein, and six accessory proteins) are encoded by shorter single guide RNA (sgRNAs) [2]. The COVID-19 disease is declared a global pandemic on March 11, 2020, by the World Health Organization (WHO), due to its severe outbreak and frightening levels of transmission. As of June 24, 2022, the COVID-19 pandemic had resulted in 547,492,681 confirmed COVID-19 cases across 193 countries, which led to 6,347,816 deaths reports to the WHO. SARS-CoV-2 is attached to the receptors angiotensin-converting enzyme 2 (ACE2) that are chiefly present in epithelial tissue of lungs, intestine, and cardiovascular systems [2]. ACE2 receptor not only acts as SARS-CoV-2 receptors but also facilitates the transmission of the virus [3]. The primary symptoms of COVID-19 include fever, dry cough, fatigue, and running nose but it is also characterized by pneumonia, lymphopenia, exhausted lymphocytes, and a cytokine storm [4]. In infected patients, formations of antibodies are reported; however, there is no clear evidence that these antibodies are protective or pathogenic against the virus to prevent reinfection.
The absence of effective clinical treatment for COVID-19 disease and its extremely contagious nature lead to the instant demand for the development of vaccines to bring the world towards normalcy. At relatively low-cost vaccines are crucial to dampen the disease load in large populations and can be proved as a potent tool to mitigate the impact of COVID-19 [4]. Both conventional and next-generation approaches are being utilized as a broad range of platforms for the development of vaccines. These approaches are viral vector (replicating and non-replicating), nucleic acid (DNA and RNA), recombinant proteins, virus-like particle, peptide, and live attenuated and inactivated viruses [5]. Apart from these above-mentioned platforms, some developing vaccines are trying to traffic the antigen into the body and others are using the body’s own cellular machinery for making the viral antigens [6].
In this article, we have discussed fourteen candidates of vaccines across two platforms that were approved for vaccination to defend against COVID-19 infection with different efficacy mentioned in Table 1. Furthermore, approximately 85 vaccines are under different phases of the clinical trial and some of them are mentioned in Table 2. On 16 March 2020, human clinical trial of the first COVID-19 vaccine candidate gets started [6]. At the end of 2021, around two billion vaccine shots are aimed to be delivered globally by WHO. To date, June 24, 2022, more than 12.02 billion vaccine doses have been administrated globally. In the landscape for the COVID-19 vaccine, numerous candidates are in their developmental phase to attain a similar goal to stop transmission and gain immunity against the virus (Table 2). This review presents an overview of the types, development, mechanisms, efficacy, and safety of the use of several vaccines of COVID-19 administrated in the different age groups of population along with their adverse effects. In this manuscript, we explored a total of fourteen vaccines developed from different conventional and novel platforms of vaccine development, which are approved and authorized for their uses.
Classification of COVID-19 Vaccines
Several vaccines to save lives against COVID-19 are already in practice and several of them are still in different stages of their development. These vaccines are all designed to prepare the body’s immune system to securely identify and block the SARS-CoV-2 infection. The vaccines are broadly classified into two platforms: (i) conventional/traditional platform and (ii) novel/next-generation platform (Fig. 2).
Conventional vaccines have proven their defense a record of defending against several infectious diseases such as poliovirus, measles, mumps, rubella, and rabies. The traditional virus platforms comprising virus-based and protein subunit vaccines have established successful results but have limited cross-protection, immunogenicity, and safety-related intricacy [21]. Conventional vaccine development strategies are based on adaptive immunity against pathogens. This platform is beneficial for immunocompromised persons and is cost-effective. However, in a pandemic situation, these classical/traditional vaccine platforms have certain limitations which make these platforms less feasible in the development of vaccines in a short period. Some disadvantages of the conventional platform are as follows: (a) for inactivated vaccines many booster doses are required for higher immunogenic response [22], (b) for production of whole-inactivated COVID-19 vaccine, safe testing is pre-requisite where a huge amount of SARS-CoV-2 virus will be required and develop the vaccine under the conditions of biosafety level 3 (BSL3) and to make sure live-attenuated viruses are secure and do not easily revert to virulent type [23] and (c) there is also a chance of contamination of virus tissue culture [24]. Therefore, there is a requirement of developing advanced vaccine strategies to conquer the downside of these traditional vaccines.
The chief prevalence of next-generation/novel platform (nucleic acid, viral vector) is its reliance on only the sequence details of the virus [25]. Novel gene-based vaccine strategies comprise nucleic acid, viral vectors, and virus-like particles vaccines [21]. Gene-based vaccines (GBVs), like viral vectors and nucleic acid vaccines, encode the genetic material and an antigen that has been delivered through the vaccination. Introducing viral protein(s) in a vaccine provide safety against infection, and rather than depending upon virus culture, the accessibility of coding sequences for this viral protein(s) is all required for the development of the vaccine. Immune responses are stimulated by successful assertion and presentation of an induced gene on antigen protein. For the production of potential GBV, the nucleic acid sequence of SARS-CoV-2 which is encoded as DNA or RNA is selected. Some advantages of GBVs are their speedy and easy production and their strong cellular and humoral responses. These inherent advantages of GBVs make facilitate fast vaccine production with high flexibility. Thus, it is obvious from the reports that next-generation platforms are being used for the production of the majority of current COVID-19 vaccines [25]. Some disadvantages of the next-generation platform comprise various allergic reactions, requirement of a cold storage facility like in mRNA vaccines, and larger production costs [25]. A number of potential COVID-19 vaccines build upon these strategies are in different stages of their development phases (Fig. 1). The vaccines are as follows:
Virus-Based Vaccines and Their Types
Virus-based vaccines are derived from classical platforms. These vaccines can consist of non-infectious live-attenuated and inactivated viruses. Several researchers are working to develop COVID-19 vaccines using the virus itself, in non-infectious forms under extensive safety testing. The virus-based vaccines are generally of two types:
Live-Attenuated Virus Vaccines
Live-attenuated virus vaccines are developed traditionally by serial passage of infectious viruses in cultured cells until it picks up mutation (Fig. 2). The mutated virus with low replication capability is selected and its pathogenic effects are reduced by some physical or chemical treatments [26]. These vaccines usually mimic natural infections that produce strong and long-lasting antibody and cell-mediated immunity. These vaccines are rarely administrated in immune-compromised patients due to the possibility of reversion of virulence properties of pathogens.
A diagrammatic representation of development and designing of different SRAS-CoV-2 vaccines; A: non-replicating viral vector vaccine; B: Replicating viral vector vaccine; C: mRNA vaccine; D: DNA vaccine; E: Protein subunit-based vaccine; F: Virus-like particle vaccine; G: Whole-inactivated virus vaccine; H: Live attenuated virus vaccine
Codagenix/Serum Institute of India developed a COVID-19 vaccine using the concept of the live-attenuated virus via codon deoptimization of which a single dose needs to be given through intranasal. The vaccine is currently in Phase 1 of its clinical trial [27]. Safety concerns related to these vaccines have led to the development of new ways to rationally attenuate an organism to minimize any tendency for reversion without compromising efficacy.
Inactivated Virus Vaccines
In the area of vaccine technology, inactivated vaccines are a conventional and time-tested method used against many emerging infections [28]. These vaccines generally include intact microorganisms that have been chemically or physically inactivated (Fig. 2). Inactivated vaccines have a comparatively fast development, making this a potential method for developing a COVID-19 vaccine. Antibody-dependent enhancement (ADE) in COVID-19 recommended that in the development of a coronavirus vaccine, special emphasis should be given to safety assessments [23]. Vaccines containing an inactivated live virus require cold chain transportation, dry structure stabilization, and a different solvent supply. These characteristics make the manufacturing process more difficult and raise the overall cost [29].
Many inactivated virus-based COVID-19 vaccines are now being evaluated in several clinical trials. The FSBSI “Chumakov Federal Scientific Centre for Research and Development of Immune‑ and Biological Products of the Russian Academy of Sciences” is working on two vaccines based on a weakened and inactivated strain of the COVID-19 virus identified in the Chumakov Centre. For a whole-inactivated vaccine against COVID-19, additional viral antigens added to the inactivated vaccine might improve the efficacy over time and might make the vaccine suitable for newly developed variations [30].
With the production of an inactivated vaccine, preclinical in vitro neutralization, and tested models, a plenty amount of virus strains are generally isolated from throat swabs of COVID-19 patients and then cultured under BSL3 situations. The three strains were isolated for BBIBP-CorV vaccine development: 19nCoV-CDC Tan-Strain03 (CQ01), 19nCoV-CDC Tan-Strain04 (QD01), and 19nCoV-CDC Tan-HB02 (HB02)19. Similarly, ten strains, CN1, CN2, CN3–CN5, and OS1 tLLoOS6, were isolated as experimental challenge strains for the development of PiCoVacc vaccine [31]. These strains were all derived from Vero cells, which have been approved by the WHO for vaccine manufacturing. The HB02 and CN2 strains, which demonstrated the best replication and formed the maximum viral yields in Vero cells, were chosen to manufacture pure inactivated BBIBP-CorV and PiCoVacc vaccines respectively. These strains were plaque filtered and passaged in Vero cells once to produce the P1 stock, which allowed for efficient growth in Vero cells and considerable productivity. On Vero cells, the P1 stock was adaptively maintained, passaged, and proliferated. Following that, additional passages were carried out to create the requisite number of stocks, with seven and four passages being carried out for the production of BBIBP-CorV and PiCoVacc vaccines, respectively. Five further passes were undertaken to produce the P10 stock, which was used to test the vaccine’s genetic stability. Deep sequencing analysis was used to examine the P10 stock, and the findings revealed that their sequence homology was more than 99.95% indicating great genetic stability. The quantity of antigen delivered to the immune system using typical viral vectors is difficult to manage. However, standard inactivation techniques include using Beta propiolactone (BPL) and/or formaldehyde to kill the virus at 2–8 °C (Fig. 2), the extracted viral solution and BPL were thoroughly mixed at 1:4000 ratios. The inactivation of 3 batches of virus eradicated viral infectivity, demonstrating the inactivation process’s high stability and reproducibility [5].
These vaccines generate a wide range of immunity and are believed to have all structural viral proteins. The virus was filtered via depth filtration and two tailored chromatography processes, culminating in a very pure vaccine formulation. Advancement and increased immunogenicity of this vaccine are achieved by adding immune-potentiators, also called vaccine adjuvants. The majority of these vaccines include aluminium hydroxide adjuvants, and one of them, VLA-2001, has two adjuvants: CpGoligodeoxynucleotides and aluminium hydroxide [32, 33].
COVAXINTM (BBV152) vaccine was manufactured by Bharat Biotech in collaboration with the Indian Council of Medical Research and the National Institute of Virology (NIV) in Bharat Biotech’s BSL-3 facility [34]. This β-propiolactone inactivated vaccine is derived from Vero Cell (CCL-81) of whole inactivated microorganisms. This vaccine is in liquid form and stored at 2–8 °C [9]. Two doses, i.e., 3 μg and 6 μg, of vaccine are used to be given at an interval of 28 days. In COVAXIN, a toll-like receptor (TLR) 7/8 agonist (imidazoquinolinone) and ViroVax’s (Kansas, USA) Alhydroxiquim-II are adjuvants used to boost immunogenic responses of the vaccine and its durability. COVAXIN was developed from 6 µg amount of viral antigen (Strain: NIV-2020–770) extracted from the intact inactivated virus [34]. In spike protein, shifting of aspartic acid to glycine at the 614th position of the amino acid causes the Asp614Gly mutation, and this mutation is reported in the NIV-2020–770 strain [34]. This 6 µg amount is raised to 0.5 ml by adding other inactive supplements such as 250 µg of aluminium hydroxide gel, 15 µg TLR 7/8 agonist (imidazoquinolinone), 2.5 mg TM 2-phenoxyethanol, and phosphate buffer saline adsorbed onto Algel [35]. A study from the NIV found that this vaccine showed a prominent role in neutralizing different COVID-19 strains [9].
BBIBP-CorV is the first Chinese COVID-19 vaccine authorized by the WHO for emergency use and is manufactured by the Beijing Bio-Institute of Biological Products [33]. This vaccine has higher stability but requires stabilization of the viral structure, which complicates the vaccine manufacturing process. This vaccine has two doses of β-propiolactone-inactivated, aluminium hydroxide-adjuvanted given at an interval of 21–28 days and shows highly effective defense, without observable antibody-dependent infection. On December 31, 2020, these doses were authorized by the National Medical Products Administration, China, and by 45 countries/jurisdictions for use in adults [36]. Ministry of health and prevention in association with the Department of Health Abu Dhabi reviewed China’s National Biotec group-Sinopharm interim analysis of the phase III trials, which shows that the BBIBP-CorV vaccines have 79% efficacy and good genetic stability that proved it as one of the most important vaccine types being developed against COVID-19 disease [14, 23]. A strong humoral immunogenic reaction was reported in 100% of vaccinated people [14]. Many pre-clinical published reports on BBIP-CorV have proved induction of increased levels of neutralizing antibodies (nAbs) against intra-tracheal COVID-19 virus in several animal models [23]. These results signify the potential of BBIBP-CorV to offer cross-protection by directing helper T lymphocytes headed for T helper type 1 (TH1) polarization [11, 37].
Sinopharm-WIBP is another β-propiolactone-inactivated vaccine candidate developed by Sinopharm and the Wuhan Institute of Biological Products, recently tested in phases III (ChiCTR2000031809) [38]. The tested injection of 2.5, 5, or 10 μg antigen adjuvanted with aluminium hydroxide was delivered in Alum adjuvant given in a prime-boost regimen of 28 days apart. Recently, phase III trials of this vaccine were completed in the United Arab Emirates and Bahrain. According to the report by JAMA, this vaccine showed 72.8% efficacy against symptomatic infections and 100% against severe COVID-19 cases [13].
Sinovac’s PiCoVacc β-propiolactone inactivated Vero cell–produced virus vaccine was developed by the Chinese company Sinovac Biotech [39]. At the initial stage, this vaccine was known as PiCoVacc but at the time of human trial, this vaccine is renamed CoronaVac. Two doses of viral protein, i.e., 3 and 6 µg were given with an alum adjuvant on groups of model animals (Rhesus monkey) at 0, 7, and 14 days. After vaccination on day 21, stronger antibody responses were observed in recipient’s administrated with a 6-µg dose [40]. Brazil published reports on January 13, 2021, presenting 50.4% efficacy towards symptomatic cases, 78% towards mild infection, and 100% effective in severe infection. In phase III clinical trial done on March 3, 202, 1 the vaccine showed 83.5% of effectiveness [41]. Both the vaccine and its unprocessed material do not need to be frozen and are kept at 2–8 °C (36–46 °F) temperature [42]. Corona Vac could stay viable in storage for up to 3 years, which might provide some benefit in the delivery of the vaccine to regions where cold chains are not available [43].
Protein-Based Vaccine
Protein-based COVID-19 vaccines are the largest category in vaccine development. These vaccines contain a viral peptide or protein antigen to carry out the immune response in the body. It is believed to be very safe and uses recent techniques for protein purification [44]. In the past, scientists have made various protein-based human vaccines like diphtheria, tetanus, and influenza virus vaccines. These vaccines reside on the microbe surface and are very effective. Primarily, these protein segments were purified from the microbes, but nowadays, they are synthesized in vitro through recombinant DNA technology. These vaccines generally use an antigen either S protein or its receptor binding domain (RBD).
The SARS-CoV-2 spike (S) glycoprotein component of the envelope of the COVID-19 virus plays an important role in the identification, neutralization of the immune response, and entry inside the host cell [45, 46]. It helps in the attachment of virus particle to the target cell and acts as the most suitable antigen for the SARS-CoV-2, which stimulates to neutralize antibodies against the foreign body (Fig. 2). The S protein contains 1273 amino acids and is made of two subunits—S1 and S2 [47]. The virus infects the host cell by endocytosis through the S protein-mediated binding to the hACE2 receptor. Therefore, the S proteins and their antigenic parts are the major targets for the drug industry of the subunit vaccine. The spike (S) protein is an active protein, consisting of two conformational states one is pre-fusion and the other is post-fusion. Therefore, the antigen must preserve its surface identity and profile of the original prefusion spike protein to spare the epitopes for stimulating an effective antibody response [48]. The S protein is an inactive precursor and the modification and insertion of polybasic RRAR furin motif in the S1/S2 cleavage site and proteolytic cleavage results in the formation of an S2 stalk that is protected across human coronaviruses [49, 50]. The S1 subunit contains the RBD and N-terminal domains. The S2 subunit contains the central helix, cytoplasmic tail, transmembrane, and fusion peptide. S-trimer is formed from three S1/S2 protomers which are associated non-covalently. The S-trimer is metastable and goes under modifications during endocytosis [45]. Rearrangement and modification help in virus-host cell interaction leading to fusion and virus entry [51].
A subunit vaccine based on recombinant peptides is essential for stimulating the long-lasting defense and curative immune response [52]. However, the subunit vaccine possesses little immune response and needs an adjuvant to enhance the vaccine-induced immune response. Therefore, the addition of an adjuvant may increase the biological half-life of antigen, or it may mitigate the immunomodulatory cytokine response and helps to disable the inadequacies of the protein subunit vaccines [53]. The receptor-binding domain (RBD) based subunit vaccine showed better immunogenicity as compared to full-length S protein. SARS-CoV-2 RBD did not harm animal immunity, whereas full-length S protein could do. Therefore, the RBD-dependent subunit vaccine may be a perfect and harmless alternative for the development of vaccines like NCT04473690 [52]. Despite this, it has been reported that S protein-based vaccines are much more effective as compared to RBD-based vaccines [54]. Presently, several vaccines are being developed using the S protein to stimulate an increased human body’s immune response [55].
Virus-like particle (VLP) vaccines show an advancement in protein subunit vaccinology and it may also consider a special set of protein subunit vaccines (Fig. 2). A VLP vaccine contains viral capsomeres which are reassembled in the form of capsids without viral genome and any other non-structural virus proteins when expressed recombinantly [44]. These noninfective particles offer a scaffold on which several antigens or epitopes can be joined, which increases cognate stimulation of B-cells and antibody response [56]. Two COVID-19 VLP vaccines are currently in clinical assessment, in which one is adjuvanted with AS03 manufactured by Medicago Inc., while the other vaccine is produced by SpyBiotech/Serum Institute of India. The triple-antigen vaccine is a multi-antigenic VLP vaccine in which recombinant spike, membrane, and envelope protein of SARS-CoV-2 have been co-expressed in genetically modified Saccharomyces cerevisiae expression platform (D-Crypt™); afterward, proteins experienced self-assembly process like VLP. Transmission electron microscopic analysis and analytical data concurrently equipped the biophysical characterization of VLP. Therefore, this prototype has already been entered into the pre-clinical trials to make a vaccine after further advancements. It is also supposed to be safe, economical, and easy for large-scale production [57].
NVX-CoV2373 is another protein-based immunogenic vaccine that primarily depends upon the recombinant expression of S-Protein [58]. The protein was firmly expressed in the Baculovirus system and the adjuvant is deployed to increase the immune response against the SARS-CoV-2 spike protein through the stimulation of neutralizing antibodies [59]. A single immunization in trials induces the anti-spike protein antibodies that prevent the expression of hACE2 receptor binding domain and bring out the SARS-CoV-2 neutralizing antibodies [60]. The prior clinical trials of the vaccine showed 89.3% efficacy with acceptable safety and immunity [15].
ZF-UZ-VAC-2001 is a protein subunit vaccine that utilizes dimer of RBD as the antigen and a non-virulent part of the COVID-19 virus. It is developed by Anhui Zhifei Longcom Biopharmaceutical Co. Ltd. and the Chinese Academy of Sciences [61]. It codes the SARS-CoV-2 RBD antigen with two copies in tandem repeat dimeric form using CHOZN CHO K1 cell line (Sigma-Aldrich Trading; Shanghai, China) as a liquid formulation. The vaccine contains 25 μg or 50 μg per 0·5 mL in a vial using aluminium hydroxide as the adjuvant. The vaccine is administered in 3 doses, the time interval between the first and second dose is 1 month while between the second and third is 4 to 6 months. After the phase 3 trials, the efficacy of the vaccine is reported to be 78% [16].
Molecular clamp stabilized spike protein vaccine with AS03 Adjuvant system will help to improve the vaccine response and lowers the amount of vaccine needed per dose [62]. Researchers are developing a molecular clamp technology-based stabilized pre-fusion, recombinant viral protein subunit vaccine, which helps to form very effective neutralizing antibodies [59]. PittCoVacc is the recombinant SARS-CoV-2 vaccine, based upon microneedle array, which includes the administration of rSARS-CoV-2 S1 and rSARS-CoV-2-S1fRS09 (recombinant immunogens). Substantial enhancement in the antigen-specific antibodies with a numerical implication was noticed in the pre-clinical trials for two weeks in the mice. Moreover, the immunogenicity of the vaccine was upheld even after the sterilization through gamma radiation. The statistically substantial titers of antibodies at a primary stage before increasing support the MNA-SARS-CoV-2 vaccine feasibility [63].
Viral Vector-Based Vaccines
This approach of using viral vectors for the development of vaccines is under implement over past years back for controlling the outbreak of various infectious diseases such as influenza A (H5N1) and Ebola virus, and even in cancers [64]. As compared to other classical approaches in which desired antigenic protein is incorporated directly into the host immune system, viral vector vaccines include the engineering of recombinant non-infectious virus consisting of antigenic DNA sequence into the host system for endogenous expression of that antigen. These viral vectors can stimulate both humoral and cellular immune systems in host cells [62]. This technique for the development of vaccines relies on the production of a recombinant virus vector by incorporating foreign antigenic protein into its genome and then injecting it into the host immune system (Fig. 2 (A and B)). The foremost step towards it is the selection of a suitable vector considering various aspects and the improvement of its genome through genetic engineering. Firstly, the structural properties of the genome of the selected virus should be known before and could be easily altered for the incorporation of desired antigenic sequence and its replication in the host cell. Secondly, it should show strong infectious responses. Thirdly, it should easily get inserted into the host genome by showing fast and efficient replication of antigen expression.
Now the next step is the selection of the target protein as antigenic sequence (full-length spike protein in case of recently developed COVID-19 vaccines like AstraZeneca, Johnson and Johnson, Sputnik V). Then the formation of the recombinant virus is achieved by the incorporation of targeted antigen protein into the vector genome. In some cases, the replication-responsive genes are removed from the virus before using it as a vector for vaccine development so that it could not show virulence after vaccination in a host. Viral vectors vaccines can be divided into two categories: non-replicating viral vector vaccines and replicating viral vector vaccines as described below:
Non-replicating Viral Vector Vaccines
In this type of vaccine development, replication-responsive genes are removed from the vector genome and insertion of an antigenic coding fragment is done. Due to the deletion of replication-responsive genes, these vectors are non-virulent which could prove to be a safer strategy for vaccine development as compared to replicating viral vectors [65]. These vectors could insert bigger size sequences but being deficient in replication genes they require higher doses to boost the host immune system [66]. The common examples of non-replicating viral vectors are Adenoviruses (e.g., human adenovirus serotype 5 (Ad5) and serotype 26 (Ad26), adeno-associated virus, alphavirus, and modified vaccinia virus Ankara. Adenoviruses are non-enveloped, double-stranded DNA viruses known to cause non-fatal infections in a variety of hosts. Their well-known application is effective in inducing immunological and gene therapy-related research studies [67]. They not only induce strong immune responses in the host but also are safe for humans. These factors have suggested them of being probable candidates for the development of vector-based vaccines [68]. Recently, several developers are working to manufacture adenovirus-based vector vaccines for the recent COVID-19 pandemic; some have completed their trial phase and are used for vaccination while others are under clinical trials.
AstraZeneca ChAdOx1 nCoV-19 (AZD1222) vaccine is developed by collaborative efforts of the University of Oxford’s Jenner Institute and AstraZeneca, UK, involving chimpanzee adenovirus as a vector. This vaccine consisted of replication-deficient chimpanzee adenovirus vector ChAdOx1 having surface glycoprotein antigenic sequence of SARS-CoV-2 [69]. The vaccination for AZD1222 is done in two separate doses with 0.5 ml of vaccine solution administered through intramuscular injection. The initial time gap between the two doses was approximately 4 to 12 weeks; however, recent reports revealed that a delayed second dose could increase the percentage of antibodies in the injected host [17]. The results of interim clinical trials on different participants confirmed that the overall safety and efficacy of AZD1222 is 70.4% [17]. Recently, European countries have temporarily suspended the use of AZD1222 under their vaccination programs as a measure of precaution, following reports of rare thromboembolic events in individuals after a few days of vaccination [70]. Subsequently, the WHO has suggested these countries continue the use of AZD1222 for vaccination as the benefits of this vaccine are overshadowed by the risks associated with it [71].
Another vaccine, Johnson and Johnson (Ad26.COV2.S) developed by Janssen Pharmaceuticals, USA utilizes Ad26 as a vector. Vector expresses full-length, stabilized SARS-CoV-2 spike protein and is lacking replication-responsive genes [18]. Ad26.COV2.S could prove strong immunogenic responses in a single dose to 90% of the vaccinated individuals despite being from different age groups [72]. The data released after its phase III clinical trial on participants from age 18 and above single dose of vaccine shows the efficacy of 66.9% in preventing moderate or critical COVID-19 [18]. The intramuscular administration of the vaccine in individuals reported an increase in antibody production after 28 days of vaccination [73].
Additionally, another vaccine that utilizes a replication-deficient human adenovirus vector is Sputnik V or Gam-COVID-Vac (rAd26-S + rAd5-S) developed by the Gamaleya National Center of Epidemiology and Microbiology in Moscow, Russia. Gam-COVID-Vac utilizes two forms of recombinant common cold adenovirus vector in two separate doses, both encoding SARS-CoV-2 spike protein as antigenic sequence [36]. The first dose is injected with recombinant adenovirus type 26 (rAd26) vector and later on after 3 weeks gap the second dose is administered with recombinant adenovirus type 5 (rAd5). The main aim of using two different vectors is to overcome the possibility of destroying the immunity with the same vector after the second dose is injected [74]. Gamaleya institute has earlier also developed the vaccine for Ebola virus disease using recombinant adenovirus 5 and vesicular stomatitis virus as vectors [75]. After the results of phase I and II trials, it was observed that vaccinated individuals have developed antibodies for SARS-CoV-2 without experiencing any adverse symptoms [19]. The interim clinical phase III trials indicated that the vaccine is 74% effective after the first dose which increased to 100% after 21 days of vaccination [19]. Therefore, it was suggested that Gam-COVID-Vac resulted in an overall vaccine efficacy of 91% against COVID-19 cases.
The collaborative works of CanSino Biologics and the Beijing Institute of Biotechnology (China) have developed Ad5-nCoV, a COVID-19 vaccine. Ad5-nCoV expresses a full-length Spike protein of SARS-CoV-2 using adenovirus type 5 replication-deficient as vector [76]. Human trials have indicated that after 28 days following a single dose of injection, strong immunogenic responses in vaccinated individuals were observed depicting an overall efficacy of 65.3% [20].
There are several manufacturers developing non-replicating vaccines that are undergoing clinical trials for COVID-19 like Shenzhen Geno-Immune Medical Institute (China), Bharat Biotech International Limited (India), ReiThera (Italy), and Ludwig-Maximilians—University of Munich (Germany).
Replicating Viral Vector Vaccines
In replicating viral vector vaccines, the viruses can be non-virulent to humans or have recombinant genomic sequences lacking virulence. The recombinant virus is prepared by inserting the antigenic coding sequences in the virus genome without disturbing replicative genes. Due to having replication responsive genes, these vectors when administered in host cells multiply the antigenic protein with each replication cycle enhancing host immunity. However, this approach could promote higher immune responses on a low dose; there is a chance of reversion of the pathogenic nature of viruses considering them a less safe option [77]. Some of the commonly used replicative viral vectors comprise the measles virus, adenovirus, and vesicular stomatitis virus.
Some of the replicating viral vector vaccine candidates are under clinical trials like the one using the recombinant vesicular stomatitis virus as vector developed by Israel Institute for Biological Research/ Weizmann Inst. of Science (Israel) [78], utilizing recombinant influenza virus under development by Beijing Wantai Biological Pharmacy, or the others under development by Jiangsu Provincial Center for Disease Prevention and Control (China) and Aivita Biomedical (USA) [79].
Nucleic Acid–Based Vaccines
Nucleic acid vaccines signify an entirely novel strategy by using genetic material—either RNA or DNA to generate strong immunogenic responses. These vaccines do not have any infectious viral protein and thus, are safer against the specific structural viral protein. Nucleic acid-based vaccines developed in a very short period, enabling fast production during pandemics [80]. The introduction of nucleic acid vaccines inside the body starts the formation of antigens. In the case of COVID-19, the antigen reported is the spike protein of the virus; this viral protein is produced and increased from genetic material by the translation process [6]. Once this genetic material is introduced into a cell, it is translated to make the antigen that will generate immunogenic responses against the pathogen. The generated immunity is strong because the antigen is formed in large quantities inside our own body. DNA and mRNA are advanced platforms due to their more effectiveness, safety, and convenience in heap production of vaccines [5]. The types of nucleic acid vaccines are described as follows:
DNA Vaccines
DNA vaccines are non-infectious and non-replicating. In the recombinant DNA vaccine approach, a DNA vector used to be transfected inside the cell nuclei where it forms a messenger RNA via transcription, and then gets translated into the antigen of the pathogen by the protein factory of the cell (Fig. 2). A purified plasmid DNA vector usually comprises RNA processing elements, a transcriptional promoter, and antigen encoding gene are encoded by lipid [81]. The injected genetic material is amplified inside the body in a large amount that gives long-term immunity but reported low immune responses in humans. DNA vaccines can integrate into host DNA and there is also a risk of its degradation by host enzymes [82]. At present, there are 4 and 14 COVID-19 DNA-based vaccines in clinical and preclinical trials respectively [83].
ZyCoV-D is the world’s first DNA vaccine against SARS-CoV-2 developed by Cadila Healthcare Ltd and approved by India’s drug regulator. The three-dose regimens of ZyCoV-D vaccine have 66% of efficacy [84]. It is also India’s first needle-free SARS-CoV-2 vaccine, administered with a disposable needle-free injector, using a narrow stream of fluid for penetration and delivery of the jab to the proper tissues under the skin. DNA plasmids were used in ZyCoV-D development that comprises genetic material. The information carried out by plasmids to the cells synthesizes the “spike protein,” which the virus utilises to latch on and infect human tissues [85]. This is the first safe vaccine tested in young individuals of the 12–18 age groups. They can be easily stored at higher temperatures between 2 and 8 °C and are stable at 25 °C, probably making their transport easy [10]. Their main drawback is tough immune responses and utilisation of three doses instead of two [86].
Modified-RNA (m-RNA) Vaccines
RNA-based vaccines have shown extraordinary assurances as of late and large numbers of them are being developed. As capable immunization against COVID-19, promising preclinical outcomes have been distributed for various RNA antibody applicants. Like DNA vaccine, the hereditary data for antigen is conveyed rather than the viral antigen, and the antigen is then translocated in the cells of the inoculated person (Fig. 2). Either a modified mRNA or a self-imitating RNA can be utilized. A high amount of antigens are needed for mRNA than for self-replicating RNA. The three significant names in the mRNA vaccine against COVID-19 are mRNA-1273, BNT162b2, and CVnCoV. These vaccines comprise an ionizable lipid that initiates ester-linkages in the lipid tails and at least one associated lipid to improve the stability of lipid nanoparticles (LNPs) that enhance endosomal escape upon cell take-up [87]. The mRNA vaccines BNT162b2 and mRNA-1273 follow a novel LNP delivery platform. They became the very first approved mRNA-based therapeutics and with a great pace, these have made it to phase III clinical testing and received the acceptance from the Food and Drug Administration and European Medicines Agency. These vaccines are currently being used to vaccinate people in the USA, Europe, and some Asian countries. Its capability to be produced in vitro is beneficial. These vaccines require storage at very low temperatures; therefore, some issues are experienced in their mass production and long stockpiling in developing countries.
In both BNT162b2 and mRNA-1273, highly-purified and N1-methyl-pseudouridine (1mΨ) have been used to modify mRNA. Conversely, CVnCoV has applied its “unmodified” mRNA which utilizes sequence engineering (e.g., reduction in uridine content), codon optimization, and select untranslated regions (UTRs). This process is followed by a strict purification protocol to eliminate dsRNA pieces, that upgrade mRNA translation, while keeping a balanced type I (Interferons) IFN activity [88, 89]. Additional base pair stability was provided in mRNA by 1mΨ nucleotide modifications, which improved its translation by giving secondary conformations [88]. Moreover, these modified secondary conformations of mRNA elevate resistance against chemical degradation, endonuclease cleavage, and mRNA translation [88]. In the production of BNT162b2 and mRNA-1273 vaccine, removal of dsRNA fragments was done along with 1mΨ replacement which firmly diminishes cytosolic RNA sensors and TLR signaling by affecting the inborn immunity generated against them [88]. In these mRNA vaccines, the formation of LNP is encoded by SM-102 and ALC-0315 (((4-Hydroxybutyl) azanediyl) bis(hexane-6,1-diyl)bis(2-hexyldecanoate)) that have a striking chemical similarity [90]. However, the mRNA-1273 vaccine was reported to surpass Onpattro’s MC3 and injected intramuscularly in model animals that proved its advanced proficiency for endosomal escape and enhanced its resistibility [87]. All things considered, it ought to be noticed that subtle primary contrasts in lipid construction and synthesis may emphatically affect the conveyance effectiveness of these vaccines. Earlier it was suspected that all three vaccine candidates contain a 50:10:38.5:1.5 mol% ratios of a lipid formulation of ionizable lipid: DSPC: cholesterol: PEG-lipid at molar and an mRNA-to-lipid ratio of 0.05 (wt/wt) [91, 92]. mRNA vaccine is coated by LNPs made up of ionizable cationic lipid encodes full-length S-2P viral antigen containing spike protein, glycoprotein, a transmembrane domain, and S1/S2 cleavage site, as well as with proline substitutions at K986P and V987P in case of mRNA-1273 and at positions 986 and 987 in CVnCoV [93].
BNT162b2 is mRNA-LNP-based vaccine developed by Pfizer, Inc., and BioNTech. BNT162b2 encodes the RBD of the full-length spike protein of the virus, modified by two proline mutations to lock it in the prefusion conformation [94], the expression of which induces immunogenic effects were observed in vaccinated people against the viral antigen. BNT162b2 administrated intramuscularly in two 30-µg doses of the diluted and thawed vaccine solution (0.3 ml each) according to the following regimen: a single dose followed by a second dose 21 days later and showed up to 95% efficacy [7]. Low temperature is required for storage of this vaccine and this creates a logistical challenge for delivery of the vaccine [95]. Phase III clinical trials (NCT04368728) result in increased viral nABs titers in sera and RBD binding IgG concentrations showed 91.3% efficacy in preventing infection within a week after the second dose [73, 96,97,98]. The BNT162b2 vaccine is being used to vaccinate people ages 12 years and older, while clinical trials to test the vaccine’s efficacy and safety in the population of < 12 years of age are in progress.
mRNA-1273 is an mRNA vaccine encapsulated with a LNP–encodes prefusion-stabilized full-length spike protein of the SARS-CoV-2 virus. It is developed by Moderna and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases, within the National Institutes of Health. The manufacture of this batch was funded by the Coalition for Epidemic Preparedness Innovations. Just after 2 months of the virus sequence identification, Moderna initiated clinical testing (NCT04283461) of mRNA-1273 vaccine. The vaccine is given as a prime-boost of two shots (100 µg, 0.5 ml each) in 28 days of intervals and can be extended up to 42 days (If necessary) [8]. During a clinical trial in humans, an increased level of nABs titers and the desired T cell responses are detected and the trial outcome shows 95% efficacy of this vaccine [98]. An exclusive attribute of this vaccine is that no preservative is required and it can be stored at a freezing temperature ranging between − 50 and − 15 °C.
CVnCoV is the third mRNA vaccine in the row which is developed and manufactured by CureVac in association with the multinational pharmaceutical company Bayer by utilizing its mRNA technology. This vaccine is intended for attaining activation of balanced immunity with maximum protein expression. the CVnCoV (CureVac) vaccine candidate comprises an “unmodified” mRNA, in which uridine content is reduced by utilizing sequence engineering, selected UTRs, and for the elimination of fragments of dsRNA a stringent purification protocol is followed [89]. It is administrated intramuscular in two doses regimen of 12 μg mRNA with 28-days apart. CVnCoV shows high immunogenic responses by inducing active humoral effects with maximum titers of virus-nABs and strong T-cell responses [93]. This mode of action involves interferon type 1 for enhanced defense mechanisms against the viral load of the body. For storing stable CVnCoV vaccine, 5 °C of temperature is required to store for up to 3 months. The vaccine can be also stored and transported for 24 h at room temperature [12]. Now this vaccine has been withdrawn, a manufacturing company in collaboration with Glaxo Smith Klein (GSK) is working on the second generation of vaccines.
Mechanism Action of Vaccine Development Platforms
Vaccination is done by injecting intramuscularly at the host body. The mRNA, inactivated, protein subunit, and virus-like particle (VLP) vaccines are transferred into cellular cytosol while DNA and viral vector vaccines moved directly into the nucleus (Fig. 3). In the case of mRNA vaccines, under a cell-free system, the mRNA is in vitro transcribed (IVT) from a DNA template directly into dendritic cells (DCs) through receptor-mediated endocytosis [99]. Cells other than showing immunological responses could also absorb vaccine mRNA and display surface spikes but dendritic cells can show more readily responses to mRNA absorption. To prevent the transfected mRNA from lysosomal degradation into host cellular machinery mRNA is escaped from entering into endosomes and released into the cytosol. This process is equipped with the ionizable LNP carrier proving to be a rate-limiting process. These mRNAs are then read by DCs ribosome and translated into viral S antigens. These antigenic proteins future undergoes post-translational modification resulting in a properly folded, fully functional protein. On the other hand, the S protein is released from the host cell proteins and exposed to prefusion-stabilized trimer constructs at the cellular surface. This S antigen, attached to the membrane of the cell, is recognized by B cells followed by internalization leading to B cell responses and neutralization of generated antibody against the S antigen. The internalized antigen peptide epitopes are moved to the endoplasmic reticulum for loading onto major histocompatibility complex (MHC) class I molecules (MHC I). The loaded MHC I-peptide antigenic epitope complexes present on the cellular surface eventually lead to induce CD8 + Tc cells that cause cell-mediated immune (CMI) response. These CMI cells remove infected cells and allow the presentation of antigenic epitopes in MHC-II complexes to CD4 + helper T cells via recycling mechanisms which elicit the humoral immune response, especially essential for B cell–mediated antibody production. Another response was observed in transfected antigen-presenting cells (APCs) which subsequently move towards the draining lymph nodes (LN) where B cells and T cells are exposed to mRNA-encoded antigens [90]. Moreover, mRNA vaccines are also used to check the innate immune system to improve their induction potential, adaptability, and immune responses specific towards the administrated antigen.
This figure shows the series of entry entries of vaccines inside the cell and their common mechanism of action followed by vaccines. (1) Vaccines are injected intra-muscularly. (2) These vaccines are transfected inside the cell (3) mRNA, inactivated, protein subunit, and virus virus-like particle (VLP) vaccines are transfected into dendritic cells (DCs) via endocytosis. (4) DNA and viral vector vaccines are transfected directly into the nucleus where they are transcribed and this transcribed RNA is then translocated to the cytoplasm. (5) Endocytosed mRNA undergoes endosomal escape and is released into the cytosol. (6) The mRNA from vaccines is translated by ribosome within the host to synthesize SARS-CoV-2 S protein. (7) Other endocytosed vaccines and formed antigen proteins are degraded by the proteasome in the cytoplasm and generated epitopes. (8) These epitopes are transported into the endoplasmic reticulum where it is loaded onto major histocompatibility complex (MHC) class I molecules (MHC I). MHC I loaded antigenic peptide epitopes presented on the host cell surface, resulting in the induction of antigen-specific CD8 + T cells. (9) Alternatively, the protein is released from the DCs. (10) The endocytosis of exogenous proteins occurs followed by MHC class II processing. (11) MHC II molecules loaded with antigenic peptide epitopes. (12) The loaded MHC II-peptide epitope complexes are presented on the surface of cells, leading to the generation of the CD4 +
Mechanism action of various types of vaccine development platforms is diagrammatically represented including the series of events that occur from vaccine injection to host immunity responses (Fig. 3). Now, in the case of vector-based vaccines, viral vector particles through receptor-mediated endocytosis move to the cytoplasm and then via nuclear membrane is transferred to the nucleus [100] (Fig. 3). The vector DNA is extrachromosomal while the antigenic protein is intracellularly transcribed and transported to the plasma membrane for its expression. The expression of antigenic protein is identified by the host immune system which further initiates immunogenic responses. The viral vector vaccines trigger innate immune cells like dendritic cells and macrophages which further stimulate multiple pattern-recognition receptors. Further, these receptors induce type I interferon secretion which signals to T cells in lymph nodes for differentiation of both CD4+ and CD8+ effector cells [101]. Inflammatory cells like cytokines and chemokines are released that cause mild side effects like pain, fever, and chills in individual post-vaccination. MHC class I molecules present the transcribed endogenously produced antigen in the host to CD8+ T-lymphocytes [102]. CD8+ cells will further trigger cell-mediated immunity by initiating the production of T-cytotoxic cells, which will destroy the future viral infection. MHC class II molecules will trigger the CD4+ T helper cells which will cause differentiation of B cell to plasma cells for producing antibodies and memory cells.
Other endocytosed vaccines like inactivated, protein subunit, and VLP vaccines cause no viral infection inside the recipient body [103] (Fig. 3). Antigen-presenting cell (APC) is a type of immune cell playing a crucial role in detecting and engulfing injected virus. The APCs split the virus into a fragment and present it on its surface. Displayed fragments are encountered by a helper T cell. Binding of these fragments with cell will cause activation of T cell. The activated T cell is responsible for the activation of other immune cells that also responds against injected vaccines. These inactivated fragments are also recognized by B cells and binding with fragments occurs due to the presence of a variable range of B cell shapes. The binding of B cells with fragments displays whole viral strain on its surface. After activation of both B and T cells, they multiplied rapidly and form antibodies having similar shape and size to their surface proteins. Cells develop immunity against a live strain of the SARS-CoV-2 virus after vaccination [104]. Antibodies formed from B cells can act only on an invasion of viral particles. However, the entrance of viral particles inside the cells is inhibited by antibodies that act on spike proteins. Inactivated vaccines can target spike proteins as well as activate T cell responses while other vaccines can only encounter spike proteins. Protein subunit vaccine contains an adjuvant that stimulates the movement of leukocyte for the enhancement of T cell, B cell, natural killer, and dendritic cells into the draining lymph nodes and boosts the biological half-life of targeted antigen [105]. In parallel to the inactivated vaccine, protein subunit vaccines with adjuvant produce chiefly antibody-mediated immunity within a week by stimulating T-cell response. Therefore, adjuvants are required for this type of vaccine to stimulate the immune response and increase vaccine efficiency.
Response of Vaccinated and Non-vaccinated Cells on SARS-CoV-2 Attack
When a non-vaccinated cell is attacked by a SARS-CoV-2 virus, the virus is perceived by the receptors present on the cell surface [106]. Then these receptors through endocytosis mediate the internalization of the viruses which further initiates the replication of new viruses [107]. The virus will hijack the host cellular machinery for the process of replication and new viruses are formed. In the infected person, there will be a hike in chemokines and cytokine levels called cytokine storm causing inflammatory responses in the body [4]. After the virus entrance into the body, the cell will fight back by initiating MHC class cells. Viruses will also cause the exhaustion of cells responsible for immunity development in the body [30]. On prolonged exposure, the viruses will attack the lungs causing severe symptoms like respiratory illness, altered gas exchange, breathing problems, and fluid accumulation [5]. Viruses will move to damage other body parts causing tissue damage and multiple organ failures ultimately to death [108].
On the other hand, when the virus attacks the vaccinated cell after the process of vaccination host body has developed cellular and humoral immunity in the body [109]. In response to the vaccine, MHC class II stimulated the production of CD4 + cells which further will help natural killer cells kill the cells infected with the virus [26]. Macrophages present in the cytosol will help in engulfing viruses in the body. Pre-developed antibodies through vaccination will start to work and will initiate the neutralization of entered viruses [110]. One of the most important aspects of pre-developed antibodies after vaccination is that they are present on the cell surface receptors, thereby blocking the virus’s entry into the cell [4]. All these factors will aid the body in recovering and fighting the infection. An illustration depicting the SARS-CoV-2 attack on a non-vaccinated and vaccinated individual is shown in (Fig. 4). The figure reveals the fluctuating cellular processes and their consequences on the immunological responses of the body in both individuals.
Emerging Vaccines and Vaccine Technologies
Vaccination is the cheapest and most efficient process that benefits the world’s health by saving 3 million lives per year [111, 112]. Therefore, to avert the COVID-19 pandemic, efforts are made for the development of an effective vaccine. Several vaccines have been developed around the world and many more are under trial process (Table 2). The development of a safe and efficient vaccine generally takes so much time as it goes through clinical trials and also needs firm regulatory approvals before it can be produced and globally distributed. The COVID-19 pandemic is affecting the world at a broad level, so the process of vaccine production and trials are being expedited [113, 114]. These vaccines are firstly pre-clinically trailed either on the human/mammalian cell cultures (in vitro) or suitable animal models (in vivo). But the novelty of the virus is the main cause that affects the determination of the best-suited animal.
Rhesus macaques, a non-human primate species, have been proposed as an animal model for pre-clinical studies which is a mandatory process for the starting of clinical trials [115]. If pre-clinical results are positive then vaccine candidates continue by passing through the three clinical trial phases to evaluate the safety and effectiveness in humans [116]. Normally the possibility of a vaccine entering into the market, taken after the pre-clinical trials by clinical testing and licensure, is less than 10%. So the production of safe and efficient vaccines against SARS-CoV-2 strains is necessary to check the future SARS-CoV-2 epidemics.
Progression of safe and broad-range vaccines against SARS-CoV-2- and other SARS-CoV-2-related viruses is a prodigious implication for averting COVID-19 [117]. In this process, various candidates have been passed for use in humans and provided a considerable shield against SARS-CoV-2. In a few months, additional trials will be done on immune responses against COVID-19.
Impact of SARS-CoV-2 Vaccine on Thrombosis
Vaccines are an essential and creditable factor in controlling the spreading of the COVID-19 pandemic and providing the individual in developing immunity against its infection [118]. Regardless of this, there have been recent studies depicting thrombosis with associated thrombocytopenia as a side effect in rare cases in some vaccinated individuals [119]. The European Medicines Agency has approved the use of adenovirus-based vaccines (ChAdOx1 nCoV-19 and Ad26.COV2.S) and mRNA-based vaccines (BNT162b2 and mRNA.1273) for preventing COVID-19 infection [120]. These vaccines are reported with some cases of thrombosis associated with severe thrombocytopenia in some individuals post-vaccination [121].
In the case of adenovirus-based vaccines, there is a possibility that adenovirus itself has induced thrombocytopenia [122]. It could be seen that the adenovirus vector increased platelet, leukocyte-derived microparticles and also trigger adenovirus fibre protein-induced cytokine activation causing thrombocytopenia [123, 124]. Moreover, adenovirus vector interaction with CD46 receptor induces thrombosis triggered by upregulation of the complement pathways [79, 125]. However, adenovirus-based vaccines and mRNA-based vaccines both utilise full-length SARS CoV-2 spike protein as antigenic sequence, and their interaction with different host membrane apparatus might cause thrombocytopenia. Cases with ChAdOx1 nCoV-19 vaccination reported heparin-induced thrombocytopenia might be due to activation of anti-platelet factor 4 by heparin [126]. Other receptors like dendritic cell-specific ICAM-3 grabbing non-integrin (DC-SIGN), C-type lectin-like receptor 2 (CLEC2) and Basigin (CD147) could induce thrombocytopenia [118]. The proposed mechanism of COVID-19 vaccine-induced thrombocytopenia has been illustrated in Fig. 5.
However, the benefits assured by these vaccines are much higher than the adversity induced with them following post-vaccination thrombocytopenia [127]. It has become inevitable to further systematically and carefully re-examine the development and action of these pre-developed vaccines in healthy or immunocompromised individuals post-vaccination.
Impact of SARS-CoV-2 on Gastrointestinal Disorders
COVID-19 was primarily considered a respiratory disease but the COVID-19 virus can cause severe systemic consequences affecting other organs of the body including the gastrointestinal (GI) tract. Some recent research have been reported that almost 40% of SARS-CoV-2 infected patients show symptoms in the GI tract including abdominal pain nausea, diarrhoea, and vomiting along with its common symptoms. Moreover, SARS-CoV-2 can vigorously infest and replicate in GI and could lead to gastrointestinal inflammation and other manifestations like increased levels of fecal calprotectin, and a systemic IL-6 response [128, 129]. Recent findings suggest that in early humoral responses, specific to SARSCoV-2 infection is the production of a large number of secretory IgA antibodies that have also shown quite a better-neutralizing action than that of IgG [130]. Short-chain fatty acid metabolites synthesized from gut bacteria play a significant role in activating the immune system and triggering inflammatory responses by boosting the functions of T helper cells, regulatory cells, Th1, and Th17 effector cells [131, 132]. Patients with COVID-19 reported gut dysbiosis where the composition of normal gut microbial flora wherein gets swapped by SARSCoV-2 [133, 134]. This gut dysbiosis could also probably be responsible for the dispersion of SARS-CoV-2 from the digestive system to other organs expressing angiotensin-converting enzyme 2 (ACE-2) and transmembrane protease serine-type 2 [135]. Infection of SARS-CoV-2 in the GI tract results in internalization of ACE-2 receptor which leads to alteration in nutrient uptake and amino acid metabolism by inhibiting the activity and expression of nutrient transporters such as the neutral amino acid transporter BAT1 (Fig. 6) [136, 137]. COVID-19 patients in severe conditions show specific cytokine storms where dangerous systemic hyperinflammatory symptoms are caused by ebullient immune responses. Cytokine storm has been identified by the increased level of several cytokines and chemokines such as interleukin-1b (IL-1 b), IL-2, IL-6, IL-7, IL-8, IL-9, IL-10, IL-17A, IL-17F, IL-23 granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and macrophage inflammatory protein-1 A (MIP1A) in serum (Fig. 6) [138]. Studies indicate an active role of Th17 lymphocytes as evidenced by elevated secretion of various pro-inflammatory cytokines and interestingly it is the same systemic inflammatory reaction observed in the case of intestinal microbial translocation [139, 140]. In various viral infections, the role of receptor-like toll-like receptor 4 (TLR-4) has been associated with the generation of inflammatory responses [140]. In the human gut, two main bacteria, i.e., Bacteroidetes and Firmicutes, are present that act as an obstacle and have many activities in the gastrointestinal tract. As about 70% of immune cells are located in the GI tract, it produces antimicrobials and plays a protective role by preventing pathogens from adhering to the intestinal membrane [141]. This gut microflora has a very crucial role in modulating the innate and adaptive immune response of the body. The composition of these microbiotas differs significantly between individuals and throughout life [142, 143]. Therefore, different immune responses have been observed in different individuals against the COVID-19 vaccine. Several researches are still going on to check the responses of interactions of COVID-19 vaccines and gut microbiota antimicrobial immune response. However, many recent reports describe the connotation between the impacts of COVID-19 vaccination and the composition of individual gut microflora. Oral vaccines induce strong humoral and cellular immune responses might be due to the increase composition of Firmicutes microbiota [144, 145]. The ability modulates the immune response by manipulating the activity and expression of gut microflora holds great potential in refining the vaccination and its effectiveness.
Diagrammatic illustration showing effects of SARS-CoV-2 on the gastrointestinal tract of the body where expressions and activities of various receptors such as angiotensin-converting enzyme 2 (ACE-2), BAT1, and Toll-like receptor have been altered. Internalization of ACE-2 receptor inhibited the activity of other receptors leading to the malabsorption of nutrients and neurological complications that directly hamper immune response and increased gastrointestinal infection. Several other factors such as interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), T helper cells (Th1 and Th17), cytokines, and chemokines such as interleukin (1, 6, 17A, 17F and 23) are some common immune responses induce by SARS-CoV-2 on the gastrointestinal tract
Concluding Remarks
In a pandemic situation, COVID-19 vaccines are developing based on two platforms, i.e., traditional platforms and next-generation platforms. Conventional vaccine development strategies are based on adaptive immunity, whereas next-generation strategies are based on adaptive as well as innate immunity against pathogens. Emerging of this pandemic lead to the accelerated development of vaccines based on novel platforms. For the very first time in humankind, mRNA vaccines are successfully administrated and used to boost immunity against infection. The lists of major approved COVID-19 vaccines developed by using these platforms are summarized in Table 1.
Virus-based vaccines platform uses a form of the virus that has been weakened or inactivated by physical and chemical methods. It does not cause infection, but still activates immunogenic responses of the body against the infection. For generating strong immunity in whole-inactivated virus vaccines adjuvants are used. These inactivated platforms are extensively utilized in this pandemic and four vaccines based on this strategy are approved and widely used all over the world. Live-attenuated virus vaccines are conventionally formed in which cell culture is treated with chemicals for degrading its virulence characteristics. Protein-based COVID-19 vaccines contain a viral peptide or protein antigen to carry out an immune response in the body. These vaccines generally use an antigen either S protein or its RBD. The S protein contains 1273 amino acids and is made of two subunits—S1 and S2. S protein–based vaccines are much more effective as compared to RBD-based vaccines. Virus-like particle (VLP) vaccines show advancement in protein subunit vaccinology and it may also consider a special set of protein subunit vaccines (Fig. 2).
Viral vector vaccines, the previously used in the development of VSV-Ebola vaccines, employ a safe virus which does not cause infection but provides a base for the development of coronavirus proteins that enhances immunity against viral infection. In viral vector vaccines, DNA recombinant technology is used to clone genes encoding that viral antigen(s). Viral vector vaccines employ replicating or non-replicating viral vectors. Replicating vector vaccines produce an antigen in the infected cells as well as in healthy cells to fight against the virus. On the other hand, non-replicating vector vaccines primarily go into cells and generate the antigen delivered by the vaccine while restricting the production of new virus particles. In nucleic acid–based vaccine, the use of mRNA has numerous advantageous features over other vaccine types. It is a non-infectious, non-integrating platform, without any reinfection or mutation. It is delivered in carrier molecule form which enhances its uptake and expression, and due to various modifications, these vaccines are more stable and efficient. There are three approved vaccines based on mRNA described briefly in this manuscript. It is convenient over traditional platforms due to its rapid development and safety. The development and mechanism of these vaccines are presented diagrammatically in Figs. 1 and 2.
After the vaccination is done, the antigenic protein enters the host cellular system. This process is mediated by surface cell receptors via the process of endocytosis. Then the antigenic protein is identified by host immunological cells like dendritic cells. These dendritic cells further stimulate the cells of MHC class I and class II, which further stimulates the differentiation of both CD4 + and CD8 + effector cells. These cells will further provide cellular and humoral immunity in a vaccinated host cell.
The COVID-19 pandemic is affecting the world at a broad level, so the process of vaccine production and trials is being expedited and to avert the COVID-19 pandemic, efforts are made for the development of an effective vaccine. Several vaccines have been developed around the world and many more are under trial process (Table 2). These vaccines are firstly pre-clinically trailed either on the human/mammalian cell cultures (in vitro) or suitable animal models (in vivo). The various vaccine candidates have been passed for use in humans and provided a considerable shield against SARS-CoV-2. In the future, additional trials will be done on immune responses against COVID-19.
Data Availability
Not applicable.
References
Dong, E., Du, H., & Gardner, L. (2020). An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases, 20, 533–534.
Kim, E., et al. (2020). Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBio Med. https://doi.org/10.1016/j.ebiom.2020.102743
Yang, X., Yu, Y., Xu, J., Shu, H., Liu, H., Wu, Y., Zhang, L., Yu, Z., Fang, M., Yu, T., & Wang, Y. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Med, 8(5), 475–481.
Su, S., Du, L., & Jiang, S. (2021). Learning from the past: development of safe and effective COVID-19 vaccines. Nature Reviews Microbiology, 19(3), 211–219. https://doi.org/10.1038/s41579-020-00462-y
Le, T. T., Andreadakis, Z., Kumar, A., Román, R. G., Tollefsen, S., Saville, M., & Mayhew, S. (2020). The COVID-19 vaccine development landscape. Nature Reviews. Drug Discovery, 19(5), 305–306.
Le, T. T., Cramer, J. P., Chen, R., & Mayhew, S. (2020). Evolution of the COVID-19 vaccine development landscape. Nature Reviews Drug Discovery, 19(10), 667–8. https://doi.org/10.1038/d41573-020-00151-8
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Perez, J. L., Marc, G. P., Moreira, E. D., Zerbini, C., & Bailey, R. (2020). Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. The New England Journal of Medicine, 383, 2603–2615. https://doi.org/10.1056/NEJMoa2034577
Thompson, M. G., Burgess, J. L., Naleway, A. L., Tyner, H. L., Yoon, S. K., Meece, J., Olsho, L. E., Caban-Martinez, A. J., Fowlkes, A., Lutrick, K., & Kuntz, J. L. (2021). Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morbidity and Mortality Weekly Report., 70(13), 495.
Sapkal, G.N., Yadav, P.D., Ella, R., Deshpande, G.R., Sahay, R.R., Gupta, N., Vadrevu, K.M., Abraham, P., Panda, S. &Bhargava, B., (2021). Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B. 1.1. 7 variant of SARS-CoV-2. Journal of Travel Medicine 28(4).
Momin, T., Kansagra, K., Patel, H., Sharma, S., Sharma, B., Patel, J., Mittal, R., Sanmukhani, J., Maithal, K., Dey, A., & Chandra, H. (2021). Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine, 38, 101020.
Xia, S., Zhang, Y., Wang, Y., Wang, H., Yang, Y., Gao, G. F., Tan, W., Wu, G., Xu, M., Lou, Z., & Huang, W. (2021). Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. The Lancet Infectious Diseases, 21(1), 39–51.
CureVac Announces Positive Results in Low Dose – 1 μg – Rabies Vaccine Clinical Phase 1 Study. (2020). https://www.curevac.com/en/2020/01/07/curevac-announces-positive-results-in-low-dose-1-%C2%B5g-rabies-vaccine-clinicalphase-1-study/
Al Kaabi, N., Zhang, Y., Xia, S., Yang, Y., Al Qahtani, M.M., et al., (2021). Effect of 2 Inactivated SARS-CoV-2 Vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA.
Huang, B., Dai, L., Wang, H., Hu, Z., Yang, X., Tan, W. and Gao, G.F., (2021). Serum sample neutralisation of BBIBP-CorV and ZF2001 vaccines to SARS-CoV-2 501Y. V2. The Lancet Microbe.
Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. https://ir.novavax.com/news-releases/newsrelease-details/novavax-covid-19-vaccine-demonstrates-893-efficacyuk-phase-3 (accessed March 10, 2021).
Yang, S., Li, Y., Dai, L., Wang, J., He, P., Li, C., Fang, X., Wang, C., Zhao, X., Huang, E. and Wu, C., (2021). Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. The Lancet Infectious Diseases.
Voysey, M., Clemens, S. A. C., Madhi, S. A., Weckx, L. Y., Folegatti, P. M., Aley, P. K., et al. (2021). Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet, 397(10269), 99–111.
Edward, H., Livingston, M.D.; Preeti, N., Malani, M.D., MSJ; C. Buddy Creech, M.D., MPH March 1, 2021. doi:https://doi.org/10.1001/jama.2021.2927 Deputy Editor, JAMA (Livingston); Associate Editor, JAMA (Malani).
Logunov, D.Y., Dolzhikova , IV &Shcheblyakov, D.V., et al. (2021). Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet.
NMPA Accepts the Application for Conditional Marketing Authorization of CanSinoBIO’s COVID-19 Vaccine ConvideciaTM. http://www.cansinotech.com/html/1///179/180/651.html (accessed March 12, 2021).
Malik, J. A., Mulla, A. H., Farooqi, T., Pottoo, F. H., Anwar, S. & Rengasamy, K. R. (2021). Targets and strategies for vaccine development against SARS-CoV-2. Biomedicine & Pharmacotherapy 137, 111254. https://doi.org/10.1016/j.biopha.2021.111254
Ura, T., Yamashita, A., Mizuki, N., Okuda, K., & Shimada, M. (2021). New vaccine production platforms used in developing SARS-CoV-2 vaccine candidates. Vaccine, 39(2), 197–201. https://doi.org/10.1016/j.vaccine.2020.11.054
Wang, H., Zhang, Y., Huang, B., Deng, W., Quan, Y., Wang, W., Xu, W., Zhao, Y., Li, N., Zhang, J., & Liang, H. (2020). Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell, 182(3), 713–721. https://doi.org/10.1016/j.cell.2020.06.008
World Health Organization MODULE 2- Types of vaccine and adverse reactions WHO. 41–42 (2018). https://www.who.int/vaccine_safety/initiative/tech_support/Part-2.pdf.
van Riel, D., & de Wit, E. (2020). Next-generation vaccine platforms for COVID-19. Nat Mat, 19(8), 810–812.
Dong, Y., Dai, T., Wei, Y., Zhang, L., Zheng, M., et al., (2020). A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduction and Targeted Therapy, 5.
Serum Institute of India Initiates Manufacturing of Codagenix’s Intranasal Live-Attenuated COVID-19 Vaccine Candidate. https://www.prnewswire.com/newsreleases/serum-institute-of-india-initiatmanufacturing-of-codagenixs-intranasal-live-attenuated-covid-19-vaccine-candidate-301135221.html (accessed December 11, 2020).
Stern, P. L. (2020). Key steps in vaccine development. Annals of Allergy, Asthma & Immunology, 125(1), 17–27. https://doi.org/10.1016/j.anai.2020.01.025
Sridhar, S., Brokstad, K. A., & Cox, R. J. (2015). Influenza vaccination strategies: Comparing inactivated and live attenuated influenza vaccines. Vaccines, 3, 373–389. https://doi.org/10.3390/vaccines3020373
Jeyanathan, M., Afkhami, S., Smaill, F., Miller, M. S., Lichty, B. D., & Xing, Z. (2020). Immunological considerations for COVID-19 vaccine strategies. Nature Reviews Immunology, 20, 615–632.
Gao, Q., Bao, L., Mao, H., Wang, L., Xu, K., Yang, M., Li, Y., Zhu, L., Wang, N., Lv, Z., & Gao, H. (2020). Development of an inactivated vaccine candidate for SARS-CoV-2. Science, 369(6499), 77–81.
Flanagan, K. L., Best, E., Crawford, N. W., et al. (2020). Progress and pitfalls in the quest for effective SARS-CoV-2 (COVID-19) vaccines. Frontiers in Immunology, 11, 579250.
World Health Organization. 2020. Draft landscape of COVID 19 candidate Vaccines. https://www.Who.Int/Who-Documents-Detail/Draft-Landscape-Of-Covid-19-Candidate-Vaccines [WWW Document] accessed 10.30.20.
Sarkale, P., Patil, S., Yadav, P., et al. (2020). First isolation of SARS-CoV-2 from clinical samples in India. Indian Journal of Medical Research, 151, 244–250.
Ella, R., Reddy, S., Jogdand, H., Sarangi, V., et al., (2021). Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. The Lancet Infectious Diseases.
Baraniuk, C., (2021). What do we know about China’s covid-19 vaccines?. bmj, 373.
Lefebvre, M., Vignier, N., Pitard, B., Botelho-Nevers, E., Cohen, R., & Epaulard, O. (2021). Covid-19 vaccines: Frequently asked questions and updated answers. Infectious Diseases Now, 51(4), 319–333.
Xia, S., Duan, K., Zhang, Y., Zhao, D., Zhang, H., Xie, Z., Li, X., Peng, C., Zhang, Y., Zhang, W., & Yang, Y. (2020). Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. Jama, 324(10), 951–960. https://doi.org/10.1001/jama.2020.15543
Parekh, N., (2020). “CoronaVac: A COVID-19 Vaccine Made From Inactivated SARS-CoV-2 Virus”. Retrieved 25 July2020
Zhang Y.-J., G. Zeng, H.-X. Pan, C.-G. Li, B. Kan, Y.-L. Hu, H.-Y. Mao, Q.-Q. Xin, K. Chu, W.-X. Han, Z. Chen, R. Tang, W.-D. Yin, X. Chen, X.-J. Gong, C. Qin, Y.-S. Hu, X.-Y. Liu,G.-L. Cui, C.-B. Jiang, H.-M. Zhang, J.-X. Li, M.-N. Yang, X.-J. Lian, Y. Song, J.-X. Lu, X.-X. Wang, M. Xu, Q. Gao, F.-C. Zhu. (2020). Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: Report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medRxiv 2020.07.31.20161216 [Preprint]. 10 August 2020. https://doi.org/10.1101/2020.07.31.20161216
AGENCIES, DAILY SABAH WITH (2020). “Turkey set to receive ‘effective’ COVID-19 vaccine amid calls for inoculation”. Daily Sabah. Retrieved 12 February 2021.
“CoronaVac: Doses will come from China on nine flights and can…” AlKhaleej Today (in Arabic). 1 November 2020. Archivedfrom the original on 16 December 2020. Retrieved 1 November2020.
Staff. (2020). “China’s Sinovac coronavirus vaccine candidate appears safe, slightly weaker in elderly”. Reuters. Archived from the original on 7 October 2020.
Li, et al. (2021). ACS Central Science, 7, 512–533.
Walls, A. C., et al. (2017). Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proceedings of the National Academy of Sciences of the United States of America, 114, 11157–11162.
Li, F., et al. (2016). Structure, function, and evolution of coronavirus spike proteins. Annual Review of Virology, 3, 237–261.
Ou, X., Liu, Y., Lei, X., et al. (2020). Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications, 1620, 11. https://doi.org/10.1038/s41467-020-15562-9
Graham, B.S., (2020). Rapid COVID-19 vaccine development. Science (May 29), 945–946.
Coutard, B., et al. (2020). The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Research, 176, 04742. https://doi.org/10.1016/j.antiviral.2020.104742
Pallesen, J., et al. (2017). Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proceedings of the National Academy of Sciences of the United States of America, 114, E7348–E7357.
Millet, J. K., & Whittaker, G. R. (2014). Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proceedings of the National academy of Sciences of the United States of America, 111, 15214–15219.
Ning, W., Jian, S., Shibo, J., & Lanying, Du. (2020). Subunit vaccines against emerging pathogenic human coronaviruses. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2020.00298
Cao, Y., Zhu, X., Hossen, M. N., Kakar, P., Zhao, Y., & Chen, X. (2018). Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nature Communication, 9(1), 1–13. https://doi.org/10.1038/s41467-018-06151-y
Krammer, F. (2020). SARS-CoV-2 vaccines in development. Nature, 586, 516–527.
Wu, D., Koganti, R., Lambe, U. P., Yadavalli, T., Nandi, S. S., & Shukla, D. (2020). Vaccines and therapies in development for SARS-CoV-2 infections. Journal of Clinical Medicine, 9(6), 1885. https://doi.org/10.3390/jcm9061885
Mohsen, M. O., Augusto, G., & Bachmann, M. F. (2020). The 3Ds in virus-like particle based-vaccines: ″Design, delivery and dynamics. Immunological Reviews, 296, 155–168.
Arora, K., Rastogi, R., et al., (2020). Multi-Antigenic Virus-like Particle of SARS CoV-2 produced in Saccharomyces cerevisiae as a vaccine candidate. Gurugram :s.n. bioRxiv (May 19). https://doi.org/10.1101/2020.05.18.099234
Coleman, C. M., Liu, Y. V., & Mu, H. (2020). Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine, 3169–3174,. https://doi.org/10.1016/j.vaccine.2014.04.016
Tu, Y.-F., Chien, C.-S., Yarmishyn, A.A., Lin, Y.-Y., Luo, Y.-H., Lin, Y.-Y., Lai, W.-Y., Yang, D.-M., Chou, S.-J., Yang, Y.-P., Wang, M.-L. & Chiou, S.-H., (2020). A review of SARS-CoV-2 and the ongoing clinical trials. International Journal of Molecular Sciences, 2657.
Novavax covid 19 vaccine trial, (2020). Clinical Trials Arena [Online]. altrialsarena.com/news/novavax-covid-19-vaccine-trial/.
An, Y., Li, S., Jin, X., Han, J.B., Xu, K., Xu, S., Han, Y., Liu, C., Zheng, T., Liu, M. and Yang, M., (2021). A tandem-repeat dimeric RBD protein-based COVID-19 vaccine ZF2001 protects mice and nonhuman primates. bioRxiv.
Lee, C. S., Bishop, E. S., Zhang, R., Yu, X., Farina, E. M., Yan, S., Zhao, C., et al. (2017). Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes & Diseases, 4, 43–63.
Kim, D., Lee, J. Y., Yang, J. S., Kim, J. W., Kim, V. N., & Chang, H. (2020). The architecture of SARS-CoV-2 transcriptome. Cell, 181(4), 914–921.
Chang, J. (2021). Adenovirus vectors: Excellent tools for vaccine development. Immune Network, 21, 1 e6. https://doi.org/10.4110/in.2021.21.e6
Bouazzaoui, A., Abdellatif, A. A., Al-Allaf, F. A., Bogari, N. M., Al-Dehlawi, S., & Qari, S. H. (2021). Strategies for vaccination: conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics, 13(2), 140. https://doi.org/10.3390/pharmaceutics13020140
Kremer, E. J. (2020). Pros and Cons of Adenovirus-Based SARS-CoV-2 Vaccines. Molecular Therapy, 28, 2303–2304. https://doi.org/10.1038/mt.2009.130
Majhen, D., Calderon, H., Chandra, N., Fajardo, C. A., Rajan, A., Alemany, R., & Custers, J. (2014). Adenovirus-based vaccines for fighting infectious diseases and cancer: Progress in the field. Human Gene Therapy, 25, 301–317.
Lasaro, M. O., & Ertl, H. C. (2009). New insights on adenovirus as vaccine vectors. Molecular Therapy, 17(8), 1333–1339. https://doi.org/10.1038/mt.2009.130
Kaur, S. P., & Gupta, V. (2020). COVID-19 vaccine: A comprehensive status report. Virus Research, 288, 198114.
Wise, J. (2021). Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ, 372. https://doi.org/10.1136/bmj.n699
WHO Statement on AstraZeneca COVID-19 vaccine safety signals. Available online: https://www.who.int/news/item/17-03-2021-who-statement-on-astrazeneca-covid-19-vaccine-safety-signals (accessed on 23 March 2021).
Sadoff, J., Le Gars, M., Shukarev, G., Heerwegh, D., Truyers, C., De Groot, A.M., Stoop, J., Tete, S., Van Damme, W., Leroux-Roels, I., et al. (2021). Interim results of a phase 1–2a Trial of Ad26.COV2.S Covid-19 vaccine. The New England Journal of Medicine, 2034201.
FDA Briefing Document, 2021. Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. In: Vaccines and related biological products advisory committee meeting; 2021. February 26, https://www.fda.gov/media/146217/download. Accessed on April 8, 2021.
Barouch, D. H., Kik, S. V., Weverling, G. J., et al. (2011). International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine, 29, 5203–5209.
Lu, S. (2009). Heterologous prime-boost vaccination. Current Opinion in Immunology, 21, 346–351.
Zhu, F. C., Li, Y. H., Guan, X. H., Hou, L. H., Wang, W. J., Li, J. X., Wu, S. P., Wang, B. S., Wang, Z., Wang, L., & Jia, S. Y. (2020). Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a doseescalation, open-label, non-randomised, first-in-human trial. The Lancet, 395(10240), 1845–1854. https://doi.org/10.1016/S0140-6736(20)31208-3
Ura, T., Okuda, K., & Shimada, M. (2014). Developments in Viral Vector-Based Vaccines. Vaccines, 2, 624–641.
Case, J. B., et al. (2020). Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis in mice. Cell Host & Microbe, 28, 465-474.e4.
Li, H., Rhee, G., Masek-Hammerman, K., Teigler, J. E., Abbink, P., & Barouch, D. H. (2021). Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. Journal of Virology, 86, 10862–10865.
Rauch, S., Gooch, K., Hall, Y., Salguero, F. J., Dennis, M. J., Gleeson, F. V., Harris, D., Ho, C., Humphries, H. E., Longet, S., & Ngabo, D. (2020). mRNA vaccine CVnCoV protects non-human primates from SARS-CoV-2 challenge infection. Biorxiv. https://doi.org/10.1101/2020.12.23.424138
Zhao, P., Ke, J. S., Qin, Z. L., Ren, H., Zhao, L. J., Yu, J. G., Gao, J., Zhu, S. Y., & Qi, Z. T. (2004). DNA vaccine of SARSCovS gene induces antibody response in mice. Acta biochimica et biophysica Sinica, 36(1), 37–41. https://doi.org/10.1093/abbs/36.1.37
Kim, J. H. J. J. (2009). DNA vaccines against Influenza viruses. Vaccines for pandemic influenza, 333, 197–210. https://doi.org/10.1007/978-3-540-92165-3
WHO. Draft landscape of COVID-19 candidate vaccines. Jan 15, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19- candidate-vaccines (accessed Jan 15, 2021).
Yadav, P., Kumar, S., Agarwal, K., Jain, M., Patil, D., Maithal, K., Mathapati, B., Giri, S., Mohandas, S., Shete, A. and Sapkal, G., (2021). Assessment of immunogenicity and protective efficacy of ZyCoV-D DNA vaccine candidates in Rhesus macaques against SARS-CoV-2 infection. BioRxiv.
Dey, A., Rajanathan, C., Chandra, H., Pericherla, H.P., Kumar, S., Choonia, H.S., Bajpai, M., Singh, A.K., Sinha, A., Saini, G. and Dalal, P., (2021). Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in Animal Models. bioRxiv.
Sahoo, J. P., Nath, S., Ghosh, L., & Samal, K. C. (2021). Concepts of immunity and recent immunization programme against COVID-19 in India. Biotics Research Today, 3(2), 103–106. https://doi.org/10.1038/s41591-021-01446-y
Hassett, K. J., Benenato, K. E., Jacquinet, E., et al. (2015). Optimization of lipid nanoparticles forintramuscular administration of mRNA vaccines Mol. Ther. Nucleic Acids, 15(2019), 1–11.
Mauger, D. M., Cabral, B. J., Presnyak, V., Su, S. V., et al. (2019). mRNA structure regulates protein expression through changes in functional half-life. Proceedings of the National Academy of Sciences of the United States of America, 116, 24075.
Lutz, J., Lazzaro, S., Habbeddine, M., et al., (2017). Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. npjVaccines 2.
Verbeke, R., Lentacker, I., De Smedt, S. C., & Dewitte, H. (2021). The dawn of mRNA vaccines: The COVID-19 case. Journal of Controlled Release, 333, 511–520.
Pardi, N., Hogan, M. J., Naradikian, M. S., Parkhouse, K., Cain, D. W., Jones, L., Moody, M. A., Verkerke, H. P., Myles, A., Willis, E., et al. (2018). Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. Journal of Experimental Medicine, 215, 1571–1588.
Orlandini von Niessen, A. G., Poleganov, M. A., Rechner, C., Plaschke, A., Kranz, L. M., Fesser, S., Diken, M., et al. (2019). Improving mRNA-based therapeutic gene delivery by expression-augmenting 3’ UTRs identified by cellular library screening. Molecular Therapy, 27, 824–836.
Rauch, S., Roth, N., Schwendt, K., Fotin-Mleczek, M., Mueller, S. O., & Petsch, B. (2020). mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines., 6(1), 1–9.
European Medicines Agency, 2020. EMA recommends first COVID-19 vaccine for authorisation in the EU.https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu
Burki T., 2021. Equitable distribution of COVID-19 vaccines. Lancet. 21(1), P33–4. Swissmedic. Comirnaty R: Fachinformation. 2020. http://www.swiss medic info.ch. Accessed 11 Feb 2021.
Mulligan, M. J., Lyke, K. E., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., et al. (2020). Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature, 586(7830), 589–593. https://doi.org/10.1038/s41586-020-2639-4
Herper, M., (2020). "Covid-19 vaccine from Pfizer and BioNTech is strongly effective, early data from large trial indicate". STAT. Archived from the original on 9 November 2020.
Corbett, K. S., Flynn, B., Foulds, K. E., Francica, J. R., et al. (2020). Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. The New England Journal of Medicine, 383(16), 1544–1555.
Chemaitelly, H., Yassine, H. M., Benslimane, F. M., Al Khatib, H. A., Tang, P., Hasan, M. R., Malek, J. A., Coyle, P., Ayoub, H. H., Al Kanaani, Z., & Al Kuwari, E. (2021). mRNA-1273 COVID-19 vaccine effectiveness against the B. 1.1. 7 and B. 1.351 variants and severe COVID-19 disease in Qatar. Nature Medicine, 27(9), 1614–1621. https://doi.org/10.1038/s41591-021-01446-y
Jones, I., & Roy, P. (2021). Sputnik V COVID-19 vaccine candidates appears safe and effective. The Lancet., 397(10275), 642–643.
Sayedahmed, E. E., Elkashif, A., Alhashimi, M., Sambhara, S., & Mittal, S. K. (2020). Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines., 8, 574.
Bolinger, B., Sims, S., Swadling, L., O’Hara, G., de Lara, C., Baban, D., et al. (2015). Adenoviral vector vaccination induces a conserved program of CD8(+) T cell memory differentiation in mouse and man. Cell Reports, 13, 1578–1588.
Heath, P. T., Galiza, E. P., Baxter, D. N., Boffito, M., Browne, D., Burns, F., Chadwick, D. R., Clark, R., Cosgrove, C., Galloway, J., & Goodman, A. L. (2021). Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. New England Journal of Medicine, 385(13), 1172–1183. https://doi.org/10.1056/NEJMoa2107659
Lazarus, J. V., Ratzan, S. C., Palayew, A., Gostin, L. O., Larson, H. J., Rabin, K., Kimball, S., & El-Mohandes, A. (2021). A global survey of potential acceptance of a COVID-19 vaccine. Nature Medicine, 27(2), 225–228.
ElBagoury, M., Tolba, M. M., Nasser, H. A., Jabbar, A., Elagouz, A. M., Aktham, Y., & Hutchinson, A. (2021). The find of COVID-19 vaccine: Challenges and opportunities. Journal of Infection and Public Health, 14(3), 389–416. https://doi.org/10.1016/j.jiph.2020.12.025
Yang, X., Yu, Y., Xu, J., Shu, H., Liu, H., Wu, Y., Zhang, L., Yu, Z., Fang, M., Yu, T., & Wang, Y. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Med, 8(5), 475–481.
Sharma, N. K., Sarode, S. C., Sarode, G., & Patil, S. (2020). Is a COVID-19 vaccine developed by nature already at work? Medical Hypotheses, 145, 110335.
Haynes, B. F., Corey, L., Fernandes, P., Gilbert, P. B., Hotez, P. J., Rao, S., Santos, M. R., Schuitemaker, H., Watson, M., & Arvin, A. (2020). Prospects for a safe COVID-19 vaccine. Science Translational Medicine, 12(568), p.eabe0948. https://doi.org/10.1126/scitranslmed.abe0948
Lurie, N., Saville, M., Hatchett, R., & Halton, J. (2020). Developing Covid-19 vaccines at pandemic speed. New England Journal of Medicine, 382, 1969–1973.
Corey, L., Mascola, J. R., Fauci, A. S., & Collins, F. S. (2020). A strategic approach to COVID-19 vaccine R&D. Science, 368(6494), 948–950.
Lipsitch, M., & Dean, N. E. (2020). Understanding COVID-19 vaccine efficacy. Science, 370(6518), 763–765.
Robertson, E., Reeve, K. S., Niedzwiedz, C. L., Moore, J., Blake, M., Green, M., Katikireddi, S. V., & Benzeval, M. J. (2021). Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain, Behavior, and Immunity., 94, 41–50.
Madhi, S. A., Baillie, V., Cutland, C. L., Voysey, M., Koen, A. L., Fairlie, L., Padayachee, S. D., Dheda, K., Barnabas, S. L., Bhorat, Q. E., & Briner, C. (2021). Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B. 1.351 variant. The New England Journal of Medicine., 384(20), 1885–1898.
Dagan, N., Barda, N., Kepten, E., Miron, O., Perchik, S., Katz, M. A., Hernán, M. A., Lipsitch, M., Reis, B., & Balicer, R. D. (2021). BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. New England Journal of Medicine, 384(15), 1412–1423.
Chung, H., Kim, H. J., Kim, J. S., Yoon, I. H., Min, B. H., Shin, J. S., Kim, J. M., Lee, W. W., & Park, C. G. (2020). CD4+/CD8+ T-cell ratio correlates with the graft fate in pig-to-non-human primate islet xenotransplantation. Xenotransplantation, 27(2), e12562.
Krause, P., Fleming, T. R., Longini, I., Henao-Restrepo, A. M., Peto, R., Dean, N. E., Halloran, M. E., Huang, Y., Fleming, T. R., Gilbert, P. B., & DeGruttola, V. (2020). COVID-19 vaccine trials should seek worthwhile efficacy. The Lancet, 396(10253), 741–743.
Theobald, N. (2020). Emerging vaccine delivery systems for COVID-19: Functionalised silica nanoparticles offer a potentially safe and effective alternative delivery system for DNA/RNA vaccines and may be useful in the hunt for a COVID-19 vaccine. Drug Discovery Today, 25(9), 1556.
Dhama, K., Sharun, K., Tiwari, R., Dadar, M., Malik, Y. S., Singh, K. P., & Chaicumpa, W. (2020). COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Human vaccines & immunotherapeutics, 16(6), 1232–1238.
Lundstrom, K., Barh, D., Uhal, B. D., Takayama, K., Aljabali, A. A., El-Aziz, A., Mohamed, T., Lal, A., Redwan, E. M., Adadi, P., & Chauhan, G. (2021). COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street? Biomolecules, 11(7), 1020.
Kleine-Tebbe, J., Klimek, L., Hamelmann, E., Pfaar, O., Taube, C., Wagenmann, M., Werfel, T., & Worm, M. (2021). Severe allergic reactions to the COVID-19 vaccine–statement and practical consequences. Allergologie select., 5, 26.
Tiede, A., Sachs, U. J., Czwalinna, A., Werwitzke, S., Bikker, R., Krauss, J. K., Donnerstag, F., Weißenborn, K., Höglinger, G., Maasoumy, B., & Wedemeyer, H. (2021). Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood, 138(4), 350–353. https://doi.org/10.1182/blood.2021011958
Morena, J., May, C., & Strohm, T. (2020). Acute thrombocytopenia after tissue plasminogen activator for stroke. Journal of Stroke and Cerebrovascular Diseases, 29, 104865.
Raddi, N., Vigant, F., Wagner-Ballon, O., Giraudier, S., Custers, J., Hemmi, S., & Benihoud, K. (2016). Pseudotyping serotype 5 adenovirus with the fiber from other serotypes uncovers a key role of the fiber protein in adenovirus 5-Induced thrombocytopenia. Human Gene Therapy, 27, 193–201.
World Health Organization, (2021). Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19): interim guidance, 19 July 2021 (No. WHO/2019-nCoV/TTS/2021.1). World Health Organization.
Oldenburg, J., Klamroth, R., Langer, F., Albisetti, M., Von Auer, C., Ay, C., Korte, W., Scharf, R. E., Potzsch, B., & Greinacher, A. (2021). Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: Guidance statement from the GTH. Hamostaseologie, 41(03), 184–189. https://doi.org/10.1055/a-1469-7481
Greinacher, A., Thiele, T., Warkentin, T. E., Weisser, K., Kyrle, P. A., & Eichinger, S. (2021). Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. New England Journal of Medicine, 384, 2092–2101.
Makris, M., Pavord, S., Lester, W., Scully, M., & Hunt, B. (2021). Vaccine-induced immune thrombocytopenia and thrombosis (VITT). Research and practice in thrombosis and haemostasis, 5(5), e12529.
Effenberger, M., Grabherr, F., Mayr, L., Schwaerzler, J., Nairz, M., Seifert, M., et al. (2020). Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut, 69(8), 1543–1544. https://doi.org/10.1136/gutjnl-2020-321388
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). ‘SARS-Cov-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Sterlin, D., Mathian, A., Miyara, M., Mohr, A., Anna, F., Claër, L., et al. (2021). Iga dominates the early neutralizing antibody response to SARS-Cov-2. Sci. Transl. Med., 13(577), eabd2223. https://doi.org/10.1126/scitranslmed.abd2223
Gonçalves, P., Araújo, J. R., & Di Santo, J. P. (2018). A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflammatory Bowel Disease, 24(3), 558–572. https://doi.org/10.1093/ibd/izx029
Li, M., van Esch, B. C., Wagenaar, G. T., Garssen, J., Folkerts, G., & Henricks, P. A. (2018). Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. European Journal of Pharmacology, 831, 52–59. https://doi.org/10.1016/j.ejphar.2018.05.003
Gou, W., Fu, Y., Yue, L., Chen, G-d., Cai, X., Shuai, M., et al., (2020). Gut Microbiota may underlie the predisposition of healthy individuals to COVID19. medRxiv 2020.04.22.20076091. doi: https://doi.org/10.1101/2020.04.22.20076091.
Gu, S., Chen, Y., Wu, Z., Chen, Y., Gao, H., Lv, L., et al. (2020). Alterations of the gut microbiota in patients with COVID-19 or H1N1 influenza. Clinical Infectious Diseases, 71(15), 2669–2678. https://doi.org/10.1093/cid/ciaa709
Kim, H. S. (2021). Do an altered gut microbiota and an associated leaky gut affect COVID-19 severity? MBio, 12(1), pp.e03022–20. https://doi.org/10.1128/mBio.03022-20
Moon, A. M., Webb, G. J., Aloman, C., et al. (2020). High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. Journal of Hepatology, S0168–8278(20), 30305–30306. https://doi.org/10.1016/j.jhep.2020.05.013
Iavarone, M., D’Ambrosio, R., Soria, A., et al. (2020). High rates of 30-day mortality in patients with cirrhosis and COVID-19. Journal of Hepatology, S0168–8278(20), 30365–30372. https://doi.org/10.1016/j.jhep.2020.06.001
Wu, D., & Yang, X. O. (2020). TH17 responses in cytokine storm of COVID19: An emerging target of JAK2 inhibitor fedratinib. Journal of Microbiology, Immunology, and Infection, 53(3), 368–370. https://doi.org/10.1016/j.jmii.2020.03.005
Cardinale, V., Capurso, G., Ianiro, G., Gasbarrini, A., Arcidiacono, P. G., & Alvaro, D. (2020). Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-Cov-2: A working hypothesis. Digestive and Liver Disease, 52(12), 1383–1389. https://doi.org/10.1016/j.dld.2020.09.009
Olejnik, J., Hume, A. J., & Mühlberger, E. (2018). Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathogens, 14(12), e1007390. https://doi.org/10.1371/journal.ppat.1007390
Falahi, S., & Kenarkoohi, A. (2022). Host factors and vaccine efficacy: Implications for COVID-19 vaccines. Journal of Medical Virology, 94(4), 1330–1335.
Huda, M. N., Lewis, Z., Kalanetra, K. M., et al. (2014). Stool microbiota and vaccine responses of infants. Pediatrics, 134(2), e362–e372.
Lynn, D. J., Benson, S. C., Lynn, M. A., & Pulendran, B. (2022). Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nature Reviews Immunology, 22(1), 33–46. https://doi.org/10.1038/s41577-021-00554-7
Cianci, R., Franza, L., Massaro, M. G., Borriello, R., De Vito, F., & Gambassi, G. (2020). The interplay between immunosenescence and microbiota in the efficacy of vaccines. Vaccines, 8(4), 636.
Harris, V. C., Armah, G., Fuentes, S., et al. (2017). Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. Journal of Infectious Diseases, 215(1), 34–41.
Author information
Authors and Affiliations
Contributions
Narsingh Bahadur Singh, Subhash Chandra Singh, and Shubhra Khare designed the manuscript. Shubhra Khare and Niharika write the manuscript. Ajey Singh and Imtiyaz Hussain analysed the data and finalized the manuscript. Shubhra Khare and Niharika prepared the first draft of the manuscript. Narsingh Bahadur Singh, Subhash Chandra Singh Ajey Singh, Niharika, Shubhra Khare, and Imtiyaz Hussain corrected and finalized the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have approved the manuscript and agree with its submission to Applied Biochemistry and Biotechnology.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khare, S., Niharika, Singh, A. et al. SARS-CoV-2 Vaccines: Types, Working Principle, and Its Impact on Thrombosis and Gastrointestinal Disorders. Appl Biochem Biotechnol 195, 1541–1573 (2023). https://doi.org/10.1007/s12010-022-04181-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04181-3