Abstract
This study was designed to identify phytocompounds from the aqueous extract of Solanum torvum unripe fruits using GC–MS analysis against breast cancer. For this, the identified phytocompounds were subjected to perform molecular docking studies to find the effects on breast cancer target protein. Pharmacokinetic properties were also tested for the identified phytocompounds to evaluate the ADMET properties. Molecular docking studies were done using docking software PyRx, and pharmacokinetic properties of phytocompounds were evaluated using SwissADME. From the results, ten best compounds were identified from GC–MS analysis against breast cancer target protein. Of which, three compounds showed very good binding affinity with breast cancer target protein. They are ergost-25-ene-3,6-dione,5,12-dihydroxy-,(5.alpha.,12.beta.) (− 7.3 kcal/mol), aspidospermidin-17-ol,1-acetyl-16-methoxy (− 6.7 kcal/mol) and 2-(3,4-dichlorophenyl)-4-[[2-[1-methyl-2-pyrrolidinyl]ethyl amino]-6-[trichloromethyl]-s-triazine (− 6.7 kcal/mol). Further, docking study was performed for the synthetic drug doxorubicin to compare the efficiency of phytocompounds. The binding affinity of ergost-25-ene-3,6-dione,5,12-dihydroxy-,(5.alpha.,12.beta.) is higher than the synthetic drug doxorubicin (− 7.2 kcal/mol), and the binding affinity of other compounds is also very near to the drug. Hence, the present study concludes that the phytocompounds from the aqueous extract of Solanum torvum unripe fruits have the potential ability to treat breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the major problems for most women worldwide. About 10% of breast cancer occurrences are due to gene mutations which are inherited [1]. The pharmacologic medications, some of which are under present use, are taxanes, doxorubicin, epothilones, vincristine, camptothecin, tamoxifen and orraloxifene to prevent breast cancer but are at high risk of developing side effects. These anticancer agents cause disruption in the normal physiological functioning of vital organs resulting in neuropathy, nephrotoxicity and chemotherapy resistance [2]. Epidemiological studies suggest that antioxidant supplements might reduce the risk of breast cancer recurrence or mortality and lower the incidence of cancers in women [3].
Hereditary mutations in the germ cell may also be a related to cancers of the breast and ovary [4, 5]. The occurrence of mutations in BRCA1 gene is a risk factor for the development of breast cancer [6, 7]. The BRCA1 protein plays a role in the differentiation of breast epithelial cells. BRCA1 is a tumor suppressor gene. The loss of BRCA1 function results in reduced acini formation and an accumulation of less differentiated cells with different proliferation properties [8].
The expression of a functional BRCA1 protein plays an important role in breast carcinogenesis [9]. BRCA1 has been mapped to long arm of chromosome 17 at 17q21. It encodes a nuclear protein of 1863 amino acids. The gene contains 24 exons, its coding region start from the middle of exon that ranges over 80 kb. It regulates transcriptional activation, maintenance of genomic integrity and stability, sex chromosome inactivation, ubiquitination, DNA repair, apoptosis, cell-cycle checkpoint control and chromosomal remodeling [10, 11]. The functional role of estrogen in breast cancer etiology and the potential integrative role between BRCA1 and the hormone synthesis are under-investigated in BRCA1 [12].
BRCA1 is identified as p53 interacting protein [13]. It interacts with RAD 51, a protein which is a major component in DNA repairing mechanism and DNA recombination. BRAC1 forms complex, which will further initiate the repairing of double-strand breaks [14, 15]. BRAC1-associated genome surveillance complex (BASC) comprises tumor suppressor gene involved in the DNA repairing process [16, 17]. Damage that occurs to BRCA1 by chance prevents the protein from DNA repair process and thereby increases the risk of developing tumor [18, 19].
Naturally occurring compounds from herbal resources with medicinal value have provided innumerable chemotherapeutics and will sustain to be an important component of drug discovery for futuristic approach [20]. There are growing evidences in the treatment of cancers by using plant origin-based phytocompounds with chemopreventive properties [21, 22]. Pharmacological studies of Solanum torvum fruits revealed antiviral, immunosecretory, antioxidant, analgesic, anti-inflammatory and anti-ulcerogenic activities [23,24,25].
Molecular docking approaches are used in modern drug design to understand drug-receptor interaction [26, 27]. Computational techniques strongly support and help the design of novel, more potent inhibitors by revealing the mechanism of drug-receptor interaction. The Swiss ADME web tool is a freely available software used to predict the physicochemical properties, absorption, distribution, metabolism, elimination and pharmacokinetic properties of molecules, which are key attempts for further clinical trials [28, 29]. The goal of ligand–protein docking is to predict the predominant binding model of a ligand with a protein of known three-dimensional structure [30].
The main objective of this study was to identify potential phytocompounds (ligands) from the aqueous extract of Solanum torvum unripe fruits for breast cancer susceptibility protein belonging to BRCA1 gene. This would be a future explorer for designing a promising novel drug, by reducing the time span of drug discovery, and could further be explored as possible therapeutic intervention for breast cancer.
Materials and Methods
Collection and Identification of Plant Material
The unripe fruits of Solanum torvum (S. torvum) were collected from in and around Kancheepuram District (12.8185°N, 79.6947°E), Tamil Nadu during the month of May to June 2018. The plant specimen was authenticated (Registration No.: PARC/2018/3855) by Dr. P. Jayaraman, Director, Plant Anatomy Research Center, Tambaram, Chennai.
Processing and Preservation of Plant Material
The unripe fruits of Solanum torvum (S. torvum) were washed with running tap water and rinsed in distilled water. The unripe fruits were chopped into small pieces and shade dried for 2 weeks for complete dryness. The dried unripe fruits were grinded to a fine powder using a mechanical grinder. The powdered material was stored in airtight containers for further use.
Preparation of Aqueous Extract (Maceration by Cold Extraction Method)
About 50 g of the dried fruit samples was separately weighed, soaked and dissolved in 600 ml of distilled water in a 1000-ml conical flask and placed for cold maceration for about 7 days at normal room temperature. The flask was tightly plugged with absorbent cotton and aluminum foil and was stirred periodically using a rotary shaker for 24 h. The extract was filtered using Whatman no. 1 filter paper. The final yield (15 g) of filtered extracts in the form of concentrated paste was used for further study.
Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
GC–MS analysis of aqueous extract of S. torvum unripe fruit was performed using GC–MS (Model: GC MS—QP 2010, Shimadzu, Japan) equipped with a VF-5 ms fused silica capillary column of 30 m length, 0.25 mm diameter and 0.25 µm film thickness. For GC–MS analysis, electron ionization system with ionization energy of 70 eV was used. The carrier gas used was helium (99.9%), at a constant flow rate of 1.2 ml/min. Injector and mass transfer line temperature were set to 280 °C and 255 °C respectively. The oven temperature was set from 50 to 250 °C at 10 °C/min for 5 min and finally raised to 300 °C for 10 min. Two microliters of the sample was injected in a split mode with a scan range of 50–1000 m/z. The total running time of GC–MS was 49 min. The relative percentage amount of each component was calculated by comparing its average peak area normalization value.
In Silico Studies
Ligand Selection
From the GC–MS analysis of the aqueous extract of S. torvum unripe fruits, 236 compounds were identified. All the compounds were analyzed. The 3D structure of all the compounds was retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and used in this study.
Selection of Target Protein
The target protein breast cancer type-1 susceptibility protein that belongs to BRCA1 gene was found from the literature. The 3D structure of this target protein was retrieved from PDB database (https://www.rcsb.org). The UniProt ID of this target protein was taken from the Uniprot database (https://www.uniprot.org/). The 3D structure of target protein breast cancer type 1 susceptibility protein for breast cancer was obtained from PDB database, and its PDB ID is 1JNX, and the UniProt ID of this target protein is P38398.
Docking Studies
Docking studies for the target protein, breast cancer type-1 susceptibility protein, BRCA1 and phytocompounds (ligands) of S. torvum fruit were performed using PyRx software [31]. All the ligands were prepared using Open Babel option in the PyRx, and the target protein was prepared using Discovery Studio 2021. The results were also analyzed using Discovery Studio 2021.
Pharmacokinetic Properties
ADMET properties were tested for the best interacted phytocompounds from the aqueous extract of S. torvum unripe fruits using SwissADME [32]. Lipophilicity (XLogP3), topological polar surface area (TPSA), solubility and hydrophobicity (Log S), carbon fraction sp3 (saturation carbons in sp3 hybridization) and rotatable bonds (flexibility) Lipinski rule, blood–brain barrier (BBB), human intestinal absorption (HIA), P-glycoprotein (PGP), cytochrome P450 inhibitor isoenzymes and skin permeation parameters were evaluated for all the compounds.
Results
GC–MS Analysis
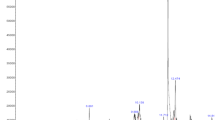
The chemical spectrum profile of S. torvum unripe fruit extract by GC–MS data was compared with the known compounds stored in the NIST library attached to the GC–MS. A total of 236 chemical structures were identified. Of which, 165 compounds were interacted with the target protein. Among these compounds, ten compounds showed very good binding affinity with the target protein. The retention time, compound name, molecular formula, molecular weight, percent area and the PubChem ID of these 10 identified phytocompounds against breast cancer target protein are shown in Table 1. The GC–MS chromatogram of the aqueous extract of S. torvum unripe fruits is shown in Fig. 1a,b.
From the results (Table 1), the best compounds ergost-25-ene-3,6-dione,5,12-dihydroxy-,(5.alpha.,12.beta.), aspidospermidin-17-ol,1-acetyl-16-methoxy and 2-(3,4-dichlorophenyl)-4-[[2-[1-methyl-2-pyrrolidinyl]ethyl amino]-6-[trichloromethyl]-S-triazine were observed in the retention time of 23.065 (0.26%), 17.722 (0.45%) and 15.286 (1.10%), respectively.
The 2D structure of the best identified phytocompounds from GC–MS analysis is shown in Table 2, and the GC–MS chromatogram of the best phytocompounds is shown in Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11.
Docking Studies
The 3D structure of target protein breast cancer type 1 susceptibility protein is depicted in Fig. 12. A total of 236 phytocompounds were identified from GC–MS analysis of the aqueous extract of S. torvum unripe fruits, in which, 165 compounds were docked with breast cancer target protein. Among these, 10 compounds showed good binding affinity with the target protein. Further, the synthetic drug doxorubicin was also docked to find the interaction and binding affinity with the target protein. The results of the docking studies with the total number of bonds, interacting amino acid residues of the target protein and the bond length are presented in Table 3.
The phytocompound ergost-25-ene-3,6-dione, 5,12-dihydroxy-, (5.alpha.,12.beta.) showed very good binding affinity (− 7.3 kcal/mol) with the amino acid residues TYR 1666, MET 1663, and LEU 1657 of target protein. The lowest binding affinity of − 5.8 kcal/mol was observed between the phytocompound 1-(2-adamantylidene) semicarbazide and the amino acid residues such as LEU 1854, GLY 1825, CYS 1828, and GLU 1829 of target protein.
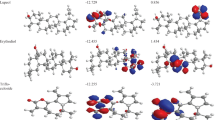
The binding affinity of − 7.2 kcal/mol was reported for the synthetic drug doxorubicin and the target protein with the amino acid residues of VAL 1740, VAL 1741, ASP 1840, THR 1852, TYR 1853, PRO 1812 and GLN 1811. The 2D and 3D interaction of the first three best interacted phytocompounds (ligands) and synthetic drug doxorubicin with the target protein is depicted in Figs. 13, 14, 15 and 16.
a 2D interaction of phytocompound 2-[3,4-dichlorophenyl]-4-[[2-[1-methyl-2-pyrrolidinyl]ethyl]amino]-6-[trichloromethyl]-S-triazine with the target protein. b 3D interaction of phytocompound 2-[3,4-dichlorophenyl]-4-[[2-[1-methyl-2-pyrrolidinyl]ethyl]amino]-6-[trichloromethyl]-S-triazine with the target protein
Physicochemical and Pharmacokinetic Properties of Phytocompounds
ADMET physicochemical and pharmacokinetic properties were tested for the ten best interacted phytocompounds of the aqueous extract of S. torvum unripe fruits and synthetic drug doxorubicin.
The compounds identified exhibited molecular weight ranging less than 500 g/mol, while for doxorubicin it was 543.5 g/mol. The X Log P3 value of 7 compounds was between the ranges of 1.00 and 5.82. The TPSA value of the compounds was within the range of 23.79 to 98.36. Log S values ranged from − 1.62 to − 5.67. Fraction C sp3 values were in the range of 0.12 to 1.00. Rotatable bonds of all the compounds were within the limit between 2 and 6 (Table 4).
The phytocompounds that best interacted obey Lipinski rule of five, while the synthetic drug doxorubicin did not obey Lipinski rule. Most of the compounds cross BBB and had high intestinal absorption. A bioavailability score of 0.55 was observed in the phytocompounds analyzed, and for doxorubicin it was 0.17 (Table 5).
The compounds ergost-25-ene-3,6-dione, 5,12-dihydroxy-, (5.alpha.,12.beta.) and 1-(2-adamantylidene) semicarbazide did not inhibit any CYP450 enzymes. The compounds aspidospermidin-17-Ol, 1-acetyl-16-methoxy and dihydroartemisinin, 10-O-(t-butyloxy) inhibited one CYP450 enzyme, CYP2D6 and CYP1A2, respectively. The compounds 2(1H)-naphthalenone, 5-[2-(3-furanyl)ethyl]octahydro-1,5,6,8a-tetramethyl and 2-butenoic acid, 2-methyl-, 4,4a,5,6,7,8,8a,9-octahydro-8a-hydroxy-3,4a,5-trimethylnaphtho[2,3-b]furan-6-yl ester inhibited the CYP450 enzyme, CYP2D6.
The phytocompounds 2-[3,4-dichlorophenyl]-4-[[2-[1-methyl-2-pyrrolidinyl]ethyl]amino]-6-[trichloromethyl]-S-triazine, 3-[(2-fluoroanilino)methyl]-5-(2-methoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione and 4H-1-benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-3,5-dihydroxy-7-methoxy inhibited 4 CYP450 enzymes.
The compound having a high negative value (log Kp) has less skin permeation ability The compounds aspidospermidin- 17-0 l, 1-acetyl-16-methoxy, 4H-1-benzopyran-4-one,2-(3,4-dimethoxyphenyl)-3,5-dihydroxy-7-methoxy and 1-(2-adamantylidene) semicarbazide have less skin permeation ability. The cytochrome and skin permeation properties are tabulated (Table 6).
Discussion
Natural products are gaining importance in the discovery of anticancer antioxidant-based lead molecules for cancer treatment [33, 34]. Computational algorithm methods are well documented in medicinal synthetic chemistry; however, their application in the field of natural phytocompounds remains scanty and unexplored. The aim of molecular docking contributes to the prediction of the ligand-receptor complex structure by computational approaches [35]. Docking mechanism executes virtual identification on library store of compounds; the results are aligned based on the scores, and structural hypothetical theories are formulated on how the ligands inhibit the target receptor, which is vital in lead enhancement [36]. The various factors affecting docking are the intramolecular forces like bond width, bond angle and dihedral angle and intermolecular forces which include electrostatic, dipolar, hydrogen bonding and hydrophobicity [37, 38].
Molecular docking studies were done for the phytocompounds of S. torvum unripe fruits with BRCA1 protein. BRCA1 plays a vital role in DNA repair, transcriptional regulation and tumor suppressor functions. The phytochemicals and commercial drug doxorubicin were docked with the active site of the target protein and help to enhance its function. However, as the commercial drug doxorubicin did not obey the Lipinski rule of five and the identified phytocompounds in our study obey the Lipinski rule of five, the present study concludes that the commercial drug doxorubicin is toxic and the identified phytocompounds in our study are non-toxic to humans. The binding affinity, different types of bonds specifically hydrogen bonds and interaction of amino acid residues with the ligand, bond length between the atom of ligand and target protein were observed. The binding affinities of the target proteins were obtained for all the phytocompounds (ligands) in terms of kcal/mol [39]. The residual interaction in the present study showed where the ligand exactly binds to particular amino acid of the protein. Out of 165 compounds docked, 10 compounds showed good binding affinity. Compounds 1, 2, 3, 4 and 5 reported binding affinity of − 7.3, − 6.7, − 6.6, − 6.5 and − 6.4 kcal/mol, respectively. The hydrogen bond indicates that the ligand had high binding affinity with the protein, and a high negative score indicates good binding affinity with the target protein [40, 41]. The binding affinity of the phytocompounds from S. torvum unripe fruits is close with the synthetic drug doxorubicin.
The important physicochemical properties, which are molecular weight, lipophilicity, polarity, solubility, saturation of carbon fractions and flexibility, represented by rotatable bonds are essential for the compounds to prove its drug-likeness [42]. Compounds which were identified with molecular weight less than or equal to 500 g/mol have the potential to be easily absorbed, diffused and transported. Lipophilic property influences the solubility, selectivity and permeability of possible drug-like compounds [43]. (XlogP3) values between the range − 0.7 and + 5.0 is proven to be a satisfying lead molecule. Compounds 1 and 6 show a very slight deviation whereas all other compounds do not deviate the range. High lipophilic nature of the compound leads to a higher rapid metabolic turnover, low solubility, negligible absorption in the intestine region and causing toxic effects to vital organs [44]. The polarity of the compounds between 20 and 130 Å, solubility not higher than 6 fits well within the acceptable range for drug-likeness. (Log S scale: insoluble < − 10 < poorly < − 6 < moderately < − 4 < soluble < − 2 < very soluble < 0 < highly soluble): The aqueous solubility is directly estimated from the compound’s molecular structure and molecular weight. Fraction of carbons in the sp3 hybridization not less than 0.25 prove to be efficient. Values of compounds 5 and 7 in the present study were less than 0.25, and the value of other compounds was above this limit. The rotatable bonds of not more than 9 determine the flexibility of the compound. The present study showed all the compounds with less than 9 rotatable bonds.
Lipinski formulated “Rule of 5” (Ro5) properties with molecular description about the compounds. The rule is helpful in the drug designing process. The present study states that the phytocompounds studied were found to be within the Lipinski’s limit range without any violation. Ro5 was used as a filter to identify compounds that have high probability of being drug candidates [45].
The gastrointestinal absorption of the identified compounds from the unripe fruit extract of S. torvum revealed the potential of being well absorbed in the gastrointestinal tract. It is suggested that these compounds are permeable from the gastrointestinal tract when they are orally administrated [46]. The BBB is a physiological barrier made up of microvascular endothelial cell layer of the brain which separates it from the blood stream. The phytocompounds were assessed for their ability to cross BBB. Eight percent of the compounds exhibit capability to cross the BBB. The penetration across BBB is a criteria for compounds targeting the central nervous system. Compounds 1, 5 and 7 did not show potential to cross BBB. This could be considered for exerting lesser adverse effects in the region of the central nervous system, whereas all other compounds had probabilities of crossing the BBB [47].
P-glycoproteins (P-gp) are compounds that act as membrane transporters in the intracellular or extracellular regions of the cell. P-glycoprotein plays a significant role in drug absorption and excretion [48]. Compound 2 to 9 are non-substrates for P-gp. This implies that the compounds if they are not a P-gp substrate would not be affected by the efflux action of P-gp, which in turn eliminates compounds from cells. Thus, only the efficacy of compound 1 and 10 has potential to be resisted in different target sites. This membrane transporter protein appears to have an impact on limiting cellular uptake of drugs from blood circulation into brain, from intestinal lumen into epithelial cells rather than on increasing the excretion of drugs from hepatic cells and renal tubules [49, 50].
All the compounds have the bioavailability value of 0.55 which implies that the compounds adhere to Lipinski rule of five and have 55% probabilities of being bioavailable. The bioavailability of drug taken orally is the fraction of the dose that reaches the bloodstream which is the crucial factor in drug designing. The bioavailability of a drug is determined mainly by gastrointestinal absorption. A drug should have good aqueous solubility for oral bioavailability and absorption [51, 52].
Cytochrome P450 monooxygenase enzyme plays an integrative role in drug metabolism and its elimination in biological systems. About 80% of the molecules in the present study identified are substrates of five isoforms CYP1A2, CYP2C19, CYP2C9, CYP2D6 and CYP3A4 [53]. The non-inhibitory action of the identified compounds against these enzymes indicated that these compounds have high probabilities of being transformed and consequently being bioavailable upon oral administration. These compounds do not inhibit the CYP450 enzymes and do not give any adverse reactions. The inhibition of the CYP isomers by these compounds can cause poor bioavailability due to metabolic derangements and toxic side effects due to their accumulation [54]. A few compounds in the present study inhibit the CYP450 enzymes and give unanticipated adverse reactions. Inhibition of these isoenzymes is a major concern of pharmacokinetics-related drug-drug interactions and its accumulation leading to toxic ADME of the drug and its metabolites [55].
The skin is a selective barrier that paves way for different compounds for its penetration. The skin permeability is a vital parameter for the assessment of compounds that might require transdermal administration. The more negative the log Kp, the less skin permeability of the molecule. All the compounds in the present study are impermeable as they are represented with the negative log Kp values [56].
Hence, the present study concludes that the phytocompounds identified from the aqueous extract of S. torvum unripe fruits using GC–MS analysis suggest the potential ability to treat breast cancer. The first six compounds indicate good binding affinity when compared with the synthetic drug doxorubicin. ADMET parameters of lead compounds using computational assessments are adapted as they circumvent the high costs, prevent unnecessary use of resources and save time. Computational methods, though not confirmatory, do provide valuable information of the most likely drug-like compounds out of an array of identified compounds [57, 58].
Ro5 is used as a tool to identify compounds that have maximum probability for being considered as a potential drug. Drugs are to be easily absorbed, metabolized and eliminated from blood stream without causing any toxic effects [59]. These drugs are distributed to the targeted site of action in the body to interact with receptor molecules. These conditions envisage that these compounds are promising therapeutic alternatives to treat metabolic and degenerative disorders. The pharmacokinetic properties analyzed were fitted well within the acceptable range for human use [60].
Data availability
All data generated or analyzed during this study are included in this article.
Change history
10 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12010-021-03749-9
References
Rebecca, L., Siegel, M. P. H., Kimberly, D., Miller, M. P. H., & AhmedinJemal, D. V. M. (2018). Cancer Statistics. CA: A Cancer Journal for Clinicians, 68(1), 7–30.
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D., Pineros, M., Znaor, A., & Bray, F. (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer, 144, 1941–1953. https://doi.org/10.1002/ijc.31937
Lester, J. (2007). Breast cancer in 2007: Incidence, risk assessment and risk reduction strategies. Clinical Journal of Oncology Nursing, 11, 619–622.
Mullan, P. B., Quinn, J. E., & Harkin, D. P. (2006). The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene, 25, 5854–5863.
Zhang, J., & Powell, S. N. (2005). The role of the BRCA1 tumor suppressor in DNA double strand break repair. Molecular Cancer Research, 3, 531–539.
Miki, Y., Swensen, J., Shattuck-Eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S., Liu, Q., Cochran, C., Bennett, L. M., & Ding, W. A. (1994). Strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science, 266, 66–71.
Burga, L. N., Tung, N. M., & Troyan, S. L. (2009). Altered proliferation and differentiation properties of primary mammary epithelial cells from BRCA1 mutation carriers. Cancer Research, 69, 1273–1278.
Pettapiece-Phillips, R., Narod, S. A., & Kotsopoulos, J. (2015). The role of body size and physical activity on the risk of breast cancer in BRCA mutation carriers. Cancer Causes and Control, 26(3), 333–344.
Lafta, I. J., & Alkaabawi, N. A. M. (2018). BRCA1 is overexpressed in breast cancer cell lines and is negatively regulated by E2F6 in normal but not cancerous breast cells. Journal of Pharmaceutical, Chemical and Biological Sciences, 5(4), 289–296.
Zhang, S., Ma, J., & Wu, J. (2009). PALB2 links BRCA1 and BRCA2 in the DNA damage response. Current Biology, 19, 524–529.
Somasundaram, K. (2003). Breast cancer gene 1 (BRCA1): Role in cell cycle regulation and DNA repair–perhaps through transcription. Journal of Cellular Biochemistry, 88, 1084–1091.
Huyton, T., Bates, P. A., Zhang, X., Sternberg, M. J., & Freemont, P. S. (2000). The BRCA1 C- terminal domain: Structure and function. Mutation Research, 460, 319–332.
Williams, R. S., & Glover, J. N. (2003). Structural consequences of a cancer causing BRCA1-BRCT missense mutation. Journal of Biological Chemistry, 278, 2630–2635.
Ekblad, C. M., Wilkinson, H. R., Schymkowitz, J. W., Rousseau, F., Freund, S. M., & Itzhaki, L. S. (2002). Characterisation of the BRCT domains of the breast cancer susceptibility gene product BRCA1. Journal of Molecular Biology, 320, 431–442.
Chen, A., Kleiman, F. E., & Manley, J. L. (2002). Autoubiquitination of the BRCA1- BARD1 RING ubiquitin ligase. Journal of Biological Chemistry, 277, 22085–22092.
Starita, L. M., Machida, Y., & Sankaran, S. (2004). BRCA1-dependent ubiquitination of tubulin regulates centrosome number. Molecular Cell Biology, 24, 8457–8466.
Fabbro, M., Savage, K., & Hobson, K. (2004). BRCA1-BARD1 complexes are required for p53 phosphorylation and a G1/S arrest following ionizing radiation-induced DNA damage. Journal of Biological Chemistry, 279, 31251–31258.
Vallon-Christersson, J., Cayanan, C., Haraldsson, K., Loman, N., Bergthorsson, J. T., Brondum-Nielsen, K., Gerdes, A. M., Moller, P., Kristoffersson, U., Olsson, H., Borg, A., & Monteiro, A. N. (2001). Functional analysis of BRCA1 C-terminal missense mutations identified in breast and ovarian cancer families. Human Molecular Genetics, 10, 34–41.
Hosford, S. R., & Miller, T. W. (2014). Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/ STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmacogenomics and Personalized Medicine, 7, 203–215.
Newman, D. J., & Cragg, G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products, 79(3), 629–661.
Sulejman, R. (2010). Wild medicinal plants and their usage in traditional human therapy. Journal of Medicinal Plants Research, 4(11), 1003–1027.
Hosseini, A., & Ghorbani, A. (2015). Cancer therapy with phytochemicals: Evidence from clinical studies. Avicenna Journal of Phytomedicine, 5(2), 84–97.
Sivapriya, M., & Srinivas, L. (2007). Isolation and purification of a novel antioxidant protein from the water extract of Sundakai (Solanum torvum) seeds. Food Chemistry, 104, 510–518.
Chah, K. F., Muko, K. N., & Oboegbulem, S. I. (2000). Antimicrobial activity of methanolic extract of Solanum torvum fruit. Fitoterapia, 71, 187–189.
Arthan, D., Svasti, J., Kittakoop, P., Pittayakhachonwut, D., Tanticharoen, M., & Hebtaranonth, Y. (2002). Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochemistry, 59, 459–478.
Mahto, M. K., Yellapu, N. K., Kilare, R. B., Chamarthi, N. R., & Bhaskar, M. (2014). Molecular designing and in silico evaluation of darunavir derivatives as anticancer agents. Bioinformation, 10(4), 221–226.
Butler, M. S. (2004). The role of natural product chemistry in drug discovery. Journal of Natural Products, 67(12), 2141–2153.
Algahtani, S. (2017). In silico ADME-Tox modeling: Progress and prospects. Expert Opinion Drug Metabolism Toxicology, 13, 1147–1158.
Ghosh, J., Lawless, M.S., Waldman, M., Gombar, V., & Fraczkiewicz, R. (2016). Modeling ADMET in silico methods for predicting drug toxicity. Methods in Molecular Biology, 63–83.
Megana, K. S. N. M., & Suneetha, Y. (2019). In-silico molecular screening of natural plant products for the identification of novel potential chemotherapeutic agents against breast cancer. International Journal of Pharmaceutical Sciences and Research, 10(10), 4546–4651. https://doi.org/10.13040/IJPSR.0975-8232.10(10).4546-51
Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455–461.
Daina, A., Michielin, O., & Zoete, V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Science Reports. 7, No. 42717.
Shapira, N. (2017). The potential contribution of dietary factors to breast cancer prevention. European Journal of Cancer Prevention, 26(5), 385–395.
Rossi, R. E., Pericleous, M., Mandair, D., Whyand, T., & Caplin, M. E. (2014). The role of dietary factors in the prevention and progression of breast cancer. Anticancer Research, 34(12), 6861–6875.
Muthusamy, K., Kirubakaran, P., Singh, D., Nagamani, S., & Sindhu, S. (2011). Molecular docking studies of bitter melon compounds against BRCA1 protein. Journal of Pharmacy Research, 4(2), 388–390.
Feinstein, W. P., & Brylinski, M. (2015). Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets. Journal of Cheminformatics, 7, 18–25.
Kerns, E. H., & Di, L. (2003). Pharmaceutical profiling in drug discovery. Drug Discovery Today, 8, 316–323.
Duran-Iturbide, N. A., Diaz-Eufracio, B. I., & Jose, L.-F. (2020). In silico ADME/Tox profiling of natural products: A focus on BIOFACQUIMACS. ACS Omega, 5, 16076–16084.
Valeriote, F., Grieshaber, C. K., Media, J., Pietraszkewics, H., & Hoftmann, J. (2002). Discovery and development of anticancer agents from plants. Journal of Experimental Therapeutics and Oncology, 2, 228–236.
Stenberg, P., Bergstrom, C. A., Luthman, K., & Artursson, P. (2002). Theoretical predictions of drug absorption in drug discovery and development. Clinical Pharmacokinetics, 41, 877–899.
Issa, N. T., Wathieu, H., Ojo, A., Byers, S. W., & Dakshanamurthy, S. (2017). Drug metabolism in preclinical drug development: A survey of the discovery process, toxicology, and computational tools. Current Drug Metabolism, 18, 556–565.
Hodgson, J. (2001). ADMET—Turning chemicals into drugs. Nature and Biotechnology, 19, 722–726.
Muegge, I., Heald, S. L., & Brittelli, D. (2001). Simple selection criteria for drug-like chemical matter. Journal of Medicinal Chemistry, 44, 1841–1846.
Arnott, J. A., & Planey, S. L. (2012). The influence of lipophilicity in drug discovery and design. Expert Opinion on Drug Discovery, 7, 863–875.
Lipinski, C. A., Lombardo, F., Dominy, B. W., & Feeney, P. J. (2012). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advances in Drug Delivery Reviews, 64, 4–17.
Pires, D. E., Kaminskas, L. M., & Ascher, D. B. (2018). Prediction and optimization of pharmacokinetic and toxicity properties of the ligand. Methods in Molecular Biology, 1762, 271–284.
Lin, J., Sahakian, D. C., Morais, S. M., Xu, J. J., Polzer, R. J., & Winter, S. M. (2003). The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Current Topics in Medicinal Chemistry, 3, 1125–1154.
El-Kattan, A., & Varma, M. (2012). Oral absorption, intestinal metabolism and human oral bioavailability. Topics on Drug Metabolism, 10, 31087–31097.
Hunter, J., & Hirst, B. H. (1997). Intestinal secretion of drugs: The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Advances in Drug Delivery Reviews, 25, 129–157.
Suzuki, H., & Sugiyama, Y. (2000). Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. European Journal of Pharmaceutical Science, 12, 3–12.
Levin, G. M. (2012). P-glycoprotein: Why this drug transporter may be clinically important. Current Psychiatry, 11, 38–40.
Veber, D. F., Johnson, S. R., Cheng, H. Y., Smith, B. R., Ward, K. W., & Kopple, K. D. (2002). Molecular properties that influence the oral bioavailability of drug candidates. Journal of Medicinal Chemistry, 45, 2615–2623.
Stegemann, S., Leveiller, F., Franchi, D., De Jong, H., & Linden, H. (2007). When poor solubility becomes an issue: From early stage to proof of concept. European Journal of Pharmaceutical Science, 31, 249–261.
Srimai, V., Ramesh, M., Parameshwar, K. S., & Parthasarathy, T. (2013). Computer-aided design of selective cytochrome P450 inhibitors and docking studies of alkyl resorcinol derivatives. Medicinal Chemistry Research, 22, 5314–5323.
Wang, Y., Xing, J., Xu, Y., Zhou, N., Peng, J., Xiong, Z., Liu, X., Luo, X., Luo, C., Chen, K., & Zheng, M. (2015). In silico ADMET modelling for rational drug design. Questions and Reviews in Biophysics, 48, 488–515.
Raies, A. B., & Bajic, V. B. (2016). In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdisciplinary Review in Computational. Molecular Science, 6, 147–172.
Maliehe, T. S., Tsilo, P. H., & Shandu, J. S. (2020). Computational evaluation of ADMET properties and bioactive score of compounds from Encephalartos ferox. Pharmacognosy Journal, 12(6), 1357–1362.
Dong, J., Wang, N. N., Yao, Z. J., Zhang, L., Cheng, Y., Ouyang, D., Lu, A. P., & Cao, D. S. (2018). ADMET lab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. Journal of cheminformatics, 10, 29–35.
Radchenko, E. V., Dyabina, A. S., Palyulin, V. A., & Zefirov, N. S. (2016). Prediction of human intestinal absorption of drug compounds. Russian Chemistry Bulletin., 65, 576–580.
Honorio, K. M., Moda, T. L., & Andricopulo, A. D. (2013). Pharmacokinetic properties and in silico ADME modeling in drug discovery. Medicinal Chemistry, 9, 163–176.
Acknowledgements
The authors wish to express sincere thanks to Nanotechnology Research Centre (NRC), SRM Institute of Science and Technology (SRMIST), Kattankulathur, Chennai for providing GC-MS Instrumentation facility. We would also like to thank Head and Project Coordinator, Iyarvi Research Centre for Bioinformatics (IRCB), Erode—638 452, Tamil Nadu, India for his support to complete docking studies successfully.
Author information
Authors and Affiliations
Contributions
R.S.: experimental design, guidance and execution of the experiment and writing the manuscript. K.R.: GC–MS analysis and computational analysis. D.S.: collection and processing of the plant material.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Yes. All authors agreed to participate in this research.
Consent for Publication
Yes. All authors have approved the last version of the manuscript for its submission.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saravanan, R., Raja, K. & Shanthi, D. GC–MS Analysis, Molecular Docking and Pharmacokinetic Properties of Phytocompounds from Solanum torvum Unripe Fruits and Its Effect on Breast Cancer Target Protein. Appl Biochem Biotechnol 194, 529–555 (2022). https://doi.org/10.1007/s12010-021-03698-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03698-3