Abstract
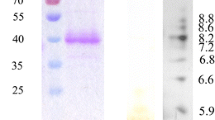
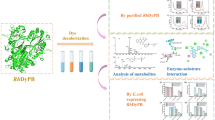
In this study, horseradish peroxidase C1A (HRP C1A) from Armoracia rusticana was expressed in Escherichia coli as an inclusion body. Subsequently, an active recombinant HRP C1A was obtained by refolding gradually using dilution-ultrafiltration. The recombinant HRP C1A was immobilized on agarose-chitosan hydrogel at 86.9 ± 2.5% of immobilization efficiency. After immobilization of the recombinant HRP C1A, the pH and temperature stability were improved and the reusability of the recombinant HPR C1A was achieved. The immobilized HRP C1A activity was retained above 80% after 6 cycles. The immobilized recombinant HRP C1A was used for the decolorization of four various dyes, including acid blue 129 (AB129), methyl blue (MB), methyl red (MR), and trypan blue (TB). The decolorization rates are all more than 70%, among which the decolorization effect of AB129 was the most significant (the decolorization rate was 76.3 ± 1.6%). Furthermore, a plausible decolorization pathway for AB129 was proposed based on the identified intermediates by ultraperformance liquid chromatography coupled with mass spectrometry (UPLC-MS). This is the first report of the putative mechanism on the decolorization of AB129 by HRP.









Similar content being viewed by others
References
Celebi, M., Kaya, M. A., Altikatoglu, M., & Yildirim, H. (2013). Enzymatic decolorization of anthraquinone and diazo dyes using horseradish peroxidase enzyme immobilized onto various polysulfone supports. Applied Biochemistry and Biotechnology, 171(3), 716–730.
Prigione, V., Tigini, V., Pezzella, C., Anastasi, A., Sannia, G., & Varese, G. C. (2008). Decolourisation and detoxification of textile effluents by fungal biosorption. Water Research, 42(12), 2911–2920.
Fatima, M., Farooq, R., Lindström, R. W., & Saeed, M. (2017). A review on biocatalytic decomposition of azo dyes and electrons recovery. Journal of Molecular Liquids, 246, 275–281.
Kariyajjanavar, P., Narayana, J., & Nayaka, Y. A. (2011). Degradation of textile wastewater by electrochemical method. Journal of Waste Water Treatment & Analysis, 02(01), 2157–7587.
Pala, A., & Tokat, E. (2002). Color removal from cotton textile industry wastewater in an activated sludge system with various additives. Water Research, 36(11), 2920–2925.
Matto, M., & Husain, Q. (2009). Decolorization of textile effluent by bitter gourd peroxidase immobilized on concanavalin a layered calcium alginate-starch beads. Journal of Hazardous Materials, 164(2–3), 1540–1546.
Amini, M., Arami, M., Mahmoodi, N. M., & Akbari, A. (2011). Dye removal from colored textile wastewater using acrylic grafted nanomembrane. Desalination, 267(1), 107–113.
Hadibarata, T., Yusoff, A. R. M., & Kristanti, R. A. (2011). Decolorization and metabolism of anthraquionone-type dye by laccase of white-rot fungi polyporus sp. S133. Water, Air, & Soil Pollution, 223(2), 933–941.
Mohan, S. V., Prasad, K. K., Rao, N. C., & Sarma, P. N. (2005). Acid azo dye degradation by free and immobilized horseradish peroxidase (HRP) catalyzed process. Chemosphere, 58(8), 1097–1105.
Gholami-Borujeni, F., Mahvi, A. H., Naseri, S., Faramarzi, M. A., Nabizadeh, R., & Alimohammadi, M. (2011). Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Research Journal of Chemistry and Environment, 15(2), 217–222.
Wasak, A., Drozd, R., Jankowiak, D., & Rakoczy, R. (2019). The influence of rotating magnetic field on bio-catalytic dye degradation using the horseradish peroxidase. Biochemical Engineering Journal, 147, 81–88.
Veitch, N. C. (2004). Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry, 65(3), 249–259.
Ferapontova, E. E., Grigorenko, V. G., Egorov, A. M., Börchers, T., Ruzgas, T., & Gorton, L. (2001). Direct electron transfer in the system gold electrode–recombinant horseradish peroxidases. Journal of Electroanalytical Chemistry, 509(1), 19–26.
Krainer, F. W., & Glieder, A. (2015). An updated view on horseradish peroxidases: recombinant production and biotechnological applications. Applied Microbiology and Biotechnology, 99(4), 1611–1625.
Passardi, F., Cosio, C., Penel, C., & Dunand, C. (2005). Peroxidases have more functions than a Swiss army knife. Plant Cell Report, 24(5), 255–265.
Grigorenko, V. G., Andreeva, I. P., Rubtsova, M. Y., & Egorov, A. M. (2015). Recombinant horseradish peroxidase: production and analytical applications. Biochemistry, 80(4), 408–416.
Felipe, L. V., & Ursula, R. (2004). Strategies for the recovery of active proteins through refolding of bacterial inclusion body proteins. Microbial Cell Factories, 3(1), 11–22.
Chang, Q., Huang, J., Ding, Y., & Tang, H. (2016). Catalytic oxidation of phenol and 2,4-dichlorophenol by using horseradish peroxidase immobilized on graphene oxide/Fe3O4. Molecules, 21(8), 1044–1054.
Yang, Y., Zhao, M., Yao, P., Huang, Y., Dai, Z., Yuan, H., & Ni, C. (2018). Comparative studies on enzyme activity of immobilized horseradish peroxidase in silica nanomaterials with three different shapes and methoxychlor degradation of vesicle-like mesoporous SiO(2) as carrier. Journal of Nanoscience Nanotechnology, 18(4), 2971–2978.
Xie, X., Luo, P., Han, J., Chen, T., Wang, Y., Cai, Y., & Liu, Q. (2019). Horseradish peroxidase immobilized on the magnetic composite microspheres for high catalytic ability and operational stability. Enzyme and Microbial Technology, 122, 26–35.
Muzzarelli, R. A. A., Boudrant, J., Meyer, D., Manno, N., DeMarchis, M., & Paoletti, M. G. (2012). Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: a tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydrate Polymers, 87(2), 995–1012.
Bilal, M., Asgher, M., Iqbal, H. M. N., Hu, H. B., & Zhang, X. C. (2017). Delignification and fruit juice clarification properties of alginate-chitosan-immobilized ligninolytic cocktail. LWT- Food Science and Technology, 80, 348–354.
Bilal, M., Rasheed, T., Zhao, Y., & Iqbal, H. M. N. (2019). Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. International Journal of Biological Macromolecules, 124, 742–749.
Iwata, H., Takagi, T., Amemiya, H., Shimizu, H., Yamashita, K., Kobayashi, K., & Akutsu, T. (1992). Agarose for a bioartificial pancreas. Journal of Biomedical Materials Research, 26(7), 967–977.
Naatsaari, L., Krainer, F. W., Schubert, M., Glieder, A., & Thallinger, G. G. (2014). Peroxidase gene discovery from the horseradish transcriptome. BMC Genomics, 15(227), 1471–1487.
Chouyyok, W., Panpranot, J., Thanachayanant, C., & Prichanont, S. (2009). Effects of pH and pore characters of mesoporous silicas on horseradish peroxidase immobilization. Journal of Molecular Catalysis B: Enzymatic, 56(4), 246–252.
Yuan, X., Tian, G., Zhao, Y., Zhao, L., Wang, H., & Ng, T. B. (2016). Degradation of dyes using crude extract and a thermostable and pH-stable laccase isolated from Pleurotus nebrodensis. Bioscience Reports, 36(4), 115–121.
Asad, S., Dabirmanesh, B., Ghaemi, N., Etezad, S. M., & Khajeh, K. (2013). Studies on the refolding process of recombinant horseradish peroxidase. Molecular Biotechnology, 54(2), 484–492.
Yang, L., Liu, X., Zhou, N., & Tian, Y. (2019). Characteristics of refold acid urease immobilized covalently by graphene oxide-chitosan composite beads. Journal of Bioscience and Bioengineering, 127(1), 16–22.
Rudolph, R., & Lilie, H. (1996). In vitro folding of inclusion body proteins. The FASEB Journal, 10(1), 49–56.
Kiefhaber, T., Rudolph, R., Kohler, H. H., & Buchner, J. (1991). Protein aggregation in vitro and in vivo: a quantitative model of the kinetic competition between folding and aggregation. Biotechnology (N Y), 9(9), 825–829.
Fang, M., & Huang, H. L. (2001). Advances in in vitro refolding of inclusion body proteins. Sheng Wu Gong Cheng Xue Bao, 17(6), 608–612.
Sun, H. F., Yang, H., Huang, W. G., & Zhang, S. J. (2015). Immobilization of laccase in a sponge-like hydrogel for enhanced durability in enzymatic degradation of dye pollutants. Journal of Colloid and Interface Science, 450, 353–360.
Nieto, M., Nardecchia, S., Peinado, C., Catalina, F., Abrusci, C., Gutiérrez, M. C., Ferrer, M. L., & del Monte, F. (2010). Enzyme-induced graft polymerization for preparation of hydrogels: synergetic effect of laccase-immobilized-cryogels for pollutants adsorption. Soft Matter, 6(15), 3533.
Bilal, M., Rasheed, T., Iqbal, H. M. N., Hu, H., Wang, W., & Zhang, X. (2017). Novel characteristics of horseradish peroxidase immobilized onto the polyvinyl alcohol-alginate beads and its methyl orange degradation potential. International Journal of Biological Macromolecules, 105(Pt 1), 328–335.
Bilal, M., Rasheed, T., Iqbal, H. M. N., Hu, H., Wang, W., & Zhang, X. (2018). Horseradish peroxidase immobilization by copolymerization into cross-linked polyacrylamide gel and its dye degradation and detoxification potential. International Journal of Biological Macromolecules, 113, 983–990.
Monier, M., Ayad, D. M., Wei, Y., & Sarhan, A. A. (2010). Immobilization of horseradish peroxidase on modified chitosan beads. International Journal of Biological Macromolecules, 46(1), 324–330.
Bilal, M., Iqbal, H. M. N., Hu, H., Wang, W., & Zhang, X. (2017). Enhanced bio-catalytic performance and dye degradation potential of chitosan-encapsulated horseradish peroxidase in a packed bed reactor system. Science of The Total Environment at ScienceDirect, 575, 1352–1360.
Krajewska, B., & Piwowarska, Z. (2005). Free vs chitosan-immobilized urease: microenvironmental effects on enzyme inhibitions. Biocatalysis and Biotransformation, 23(3–4), 225–232.
You, B. H., Li, Q. T., Dong, H., Huang, T., Cao, X. D., & Liao, H. (2018). Bilayered HA/CS/PEGDA hydrogel with good biocompatibility and self-healing property for potential application in osteochondral defect repair. Journal of Materials Science & Technology, 34(6), 1016–1025.
Illeperuma, W. R., Rothemund, P., Suo, Z., & Vlassak, J. J. (2016). Fire-resistant hydrogel-fabric laminates: a simple concept that may save lives. ACS Applied Materials & Interfaces, 8(3), 2071–2077.
Jamal, F., Qidwai, T., Singh, D., & Pandey, P. K. (2012). Biocatalytic activity of immobilized pointed gourd (Trichosanthes dioica) peroxidase-concanavalin a complex on calcium alginate pectin gel. Journal of Molecular Catalysis B: Enzymatic, 74(1–2), 125–131.
Wang, S., Liu, W., Zheng, J., & Xu, X. (2016). Immobilization of horseradish peroxidase on modified PAN-based membranes for the removal of phenol from buffer solutions. The Canadian Journal of Chemical Engineering, 94(5), 865–871.
Liu, J. Z., Wang, T. L., & Ji, L. N. (2006). Enhanced dye decolorization efficiency by citraconic anhydride-modified horseradish peroxidase. Journal of Molecular Catalysis B: Enzymatic, 41(3–4), 81–86.
Lade, H., Kadam, A., Paul, D., & Govindwar, S. (2015). Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI Journal, 14, 158–174.
Sekuljica, N. Z., Prlainovic, N. Z., Stefanovic, A. B., Zuza, M. G., Cickaric, D. Z., Mijin, D. Z., & Knezevic-Jugovic, Z. D. (2015). Decolorization of anthraquinonic dyes from textile effluent using horseradish peroxidase: optimization and kinetic study. Scientific World Journal, 10, 1155–1167.
Funding
This work was supported by the National Natural Science Foundation of China (31472003 and 31600091), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (no. 111-2-06), and the Jiangsu province “Collaborative Innovation Center for Advanced Industrial Fermentation” industry development program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, YJ., Xu, KZ., Ma, H. et al. Recombinant Horseradish Peroxidase C1A Immobilized on Hydrogel Matrix for Dye Decolorization and Its Mechanism on Acid Blue 129 Decolorization. Appl Biochem Biotechnol 192, 861–880 (2020). https://doi.org/10.1007/s12010-020-03377-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03377-9