Abstract
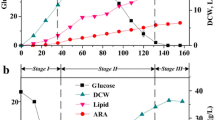
This study led in the pioneering technique incubated in a bioreactor with the forced air injection system. The purpose of this study was to establish the optimal incubation conditions for this technique. The results showed that the speed at which Inonotus obliquus was incubated with the forced air injection system was superior to that with a normal bioreactor. A nitrogen to oxygen ratio of 50:50 provided the best results with the forced air injection system, including in terms of the achievement of biomass, total triterpenes, betulinic acid content, and the scavenging activities of DPPH radicals, which reached up to 21.3 g/1000 mL, 2.1 g/1000 mL, 1.9 g/1000 mL, and 87.3%, respectively. The results showed that the bioreactor with the forced air injection system could more effectively incubate I. obliquus by using less vapor while still utilizing a model close to that of a traditional bioreactor. The innovative bioreactor fermentation model was thus more economical than the traditional bioreactor model.


Similar content being viewed by others
References
Sun, J. E., Ao, Z. H., Lu, Z. M., Xu, H. Y., Zhang, X. M., Dou, W. F., & Xu, Z. H. (2008). Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. Journal of Ethnopharmacology, 118(1), 7–13.
Handa, N., Yamada, T., & Tanaka, R. (2010). An unusual lanostane-type triterpenoid, spiroinonotsuoxodiol, and other triterpenoids from Inonotus obliquus. Phytochemistry, 71(14–15), 1774–1779.
Zheng, W., Zhang, M., Zhao, Y., Wang, Y., Miao, K., & Wei, Z. (2009). Accumulation of antioxidant phenolic constituents in submerged cultures of Inonotus obliquus. Bioresource Technology, 100(3), 1327–1335.
Solomon, P. W., & Alexander, L. W. (1999). Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Immunobiology, 19, 65–96.
Ham, S. S., Kim, S. H., Moon, S. Y., Chung, M. J., Cui, C. B., Han, E. K., Chung, C. K., & Choe, M. (2009). Antimutagenic effects of subfractions of Chaga mushroom (Inonotus obliquus) extract. Mutation Research, 672(1), 55–59.
Hu, H., Zhang, Z., Lei, Z., Yang, Y., & Sugiura, N. (2009). Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. Journal of Bioscience and Bioengineering, 107(1), 42–48.
Kim, Y. O., Han, S. B., Lee, H. W., Ahn, H. J., Yoon, Y. D., Jung, J. K., Kim, H. M., & Shin, C. S. (2005). Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sciences, 77(19), 2438–2456.
Moore, D., Robson, G. D., & Trinci, A. P. J. (2011). 21st century guidebook to fungi. Cambridge: Cambridge University Press.
Chu, S.-C., & Chen, C. (2006). Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chemistry, 98(3), 502–507.
Xu, X., & Zhu, J. (2011). Enhanced phenolic antioxidants production in submerged cultures of Inonotus obliquus in a ground corn stover medium. Biochemical Engineering Journal, 58-59, 103–109.
Jeng, K.-C., Chen, C.-S., Fang, Y.-o., Hou, R. C.-W., & Chen, Y. S. (2007). Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. Journal of Agriculture and Food Chemistry, 55, 8787–8792.
Xu, X., Quan, L., & Shen, M. (2015). Effect of chemicals on production, composition and antioxidant activity of polysaccharides of Inonotus obliquus. International Journal of Biological Macromolecules, 77, 143–150.
Zheng, W., Zhang, M., Zhao, Y., Miao, K., Pan, S., Cao, F., & Dai, Y. (2011). Analysis of antioxidant metabolites by solvent extraction from sclerotia of Inonotus obliquus (Chaga). Phytochemical Analysis, 22(2), 95–102.
Zheng, W., Miao, K., Liu, Y., Zhao, Y., Zhang, M., Pan, S., & Dai, Y. (2010). Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Applied Microbiology and Biotechnology, 87(4), 1237–1254.
Zhao, G., Yan, W., & Cao, D. (2007). Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. Journal of Pharmaceutical and Biomedical Analysis, 43(3), 959–962.
Lu, M.-J., & Chen, C. (2008). Enzymatic modification by tannase increases the antioxidant activity of green tea. Food Research International, 41(2), 130–137.
Chen, Y. S., Liu, B. L., & Chang, Y. N. (2010). Bioactivities and sensory evaluation of Pu-erh teas made from three tea leaves in an improved pile fermentation process. Journal of Bioscience and Bioengineering, 109(6), 557–563.
Zheng, W., Zhang, M., Zhao, Y., Miao, K., & Jiang, H. (2009). NMR-based metabonomic analysis on effect of light on production of antioxidant phenolic compounds in submerged cultures of Inonotus obliquus. Bioresource Technology, 100(19), 4481–4487.
Lee, K. R., Lee, J. S., Lee, S., Son, Y. K., Kim, G. R., Sim, Y. C., Song, J. E., Ha, S. J., & Hong, E. K. (2016). Polysaccharide isolated from the liquid culture broth of Inonotus obliquus suppresses invasion of B16-F10 melanoma cells via AKT/NF-kappaB signaling pathway. Molecular Medicine Reports, 14(5), 4429–4435.
Zheng, W., Zhao, Y., Zheng, X., Liu, Y., Pan, S., Dai, Y., & Liu, F. (2011). Production of antioxidant and antitumor metabolites by submerged cultures of Inonotus obliquus cocultured with Phellinus punctatus. Applied Microbiology and Biotechnology, 89(1), 157–167.
Zheng, W., Liu, Y., Pan, S., Yuan, W., Dai, Y., & Wei, J. (2011). Involvements of S-nitrosylation and denitrosylation in the production of polyphenols by Inonotus obliquus. Applied Microbiology and Biotechnology, 90(5), 1763–1772.
Xu, X., Xu, Z., Shi, S., & Lin, M. (2017). Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresource Technology, 241, 415–423.
Jinu, M. V., Gini, C. K., & Jayabaskaran, C. (2015). Modulating the biosynthsis of a bioactive steroidal saponin, cholestanol. International Journal of Pharmacy and Pharmaceutical Sciences, 7(7), 114–117.
Wang, M. Y., Zhao, Z. Z., Zhou, X., Hu, J. R., Xue, J., Liu, X., Zhang, J. S., Liu, P., & Tong, S. S. (2019). Simultaneous use of stimulatory agents to enhance the production and hypoglycaemic activity of polysaccharides from Inonotus obliquus by submerged fermentation. Molecules, 24(4400), 1–14.
Xu, X. Q., Wu, P., Wang, T. Z., Yan, L. L., Lin, M. M., & Cheb, L. (2019). Synergistic effects of surfactant-assisted biodegradation of wheat straw and production of polysaccharides by Inonotus obliquus under submerged fermentation. Bioresource Technology, 278, 43–50.
Xu, X., Wu, Y., & Chen, H. (2011). Comparative antioxidative characteristics of polysaccharide-enriched extracts from natural sclerotia and cultured mycelia in submerged fermentation of Inonotus obliquus. Food Chemistry, 127(1), 74–79.
Li, Y., Meng, S., Shi, M., Hu, X., Yang, Y., & Zhang, Z. (2016). Bioactivity evaluation of crude Polysaccharide from Rice bran fermented by Preussia aemulans and the changes in its nutritional contents. Journal of Food Biochemistry, 40(5), 664–672.
Xue, J., Tong, S. S., Wang, Z. R., & Liu, P. (2018). Chemical characterization and hypoglycaemic activities in vitro of two polysaccharides from Inonotus obliquus by submerged culture. Molecules, 23(3261), 1–14.
Cui, Y., Kim, D. S., & Park, K. C. (2005). Antioxidant effect of Inonotus obliquus. Journal of Ethnopharmacology, 96(1–2), 79–85.
Burmasova, M. A., Utebaeva, A. A., Sysoeva, E. V., & Sysoeva, M. A. (2019). Melanins of Inonotus Obliquus: bifidogenic and antioxidant properties. Biomolecules, 9(248), 1–9.
Zhao, F., Mai, Q., Ma, J., Xu, M., Wang, X., Cui, T., Qiu, F., & Han, G. (2015). Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia, 101, 34–40.
Bai, Y.-H., Feng, Y.-Q., Mao, D.-B., & Xu, C.-P. (2012). Optimization for betulin production from mycelial culture of Inonotus obliquus by orthogonal design and evaluation of its antioxidant activity. Journal of the Taiwan Institute of Chemical Engineers, 43(5), 663–669.
Xiang, Y., Xu, X., & Li, J. (2012). Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chemistry, 134(4), 1899–1905.
Woo, Y. A., Kim, H. J., Cho, J. H., & Chung, H. (1999). Discrimination of herbal medicines according to geographical origin with near infrared reflectance spectroscopy and pattern recognition techniques. Journal of Pharmaceutical and Biomedical Analysis, 21(2), 407–413.
Karoui, R., Dufour, É., Pillonel, L., Schaller, E., Picque, D., Cattenoz, T., & Bosset, J. (2005). The potential of combined infrared and fluorescence spectroscopies as a method of determination of the geographic origin of Emmental cheeses. International Dairy Journal, 15, 287–298.
Funding
The authors thank the financial support for the Ministry of Science and Technology, Taiwan, ROC (MOST104-2262-E-241-004-CC3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, HJ., Chen, YS., Liu, SL. et al. The Influence of Submerged Fermentation of Inonotus obliquus with Control Atmosphere Treatment on Enhancing Bioactive Ingredient Contents. Appl Biochem Biotechnol 191, 412–425 (2020). https://doi.org/10.1007/s12010-020-03273-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03273-2