Abstract
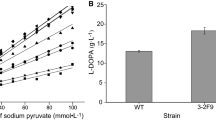
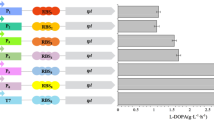
L-DOPA (L-dihydroxyphenylalanine) is a promising drug for Parkinson’s disease and thereby has a growing annual demand. Tyrosine phenol-lyase (TPL)–based catalysis is considered to be a low-cost yet efficient route for biosynthesis of L-DOPA. TPL is a tetrameric enzyme that catalyzes the synthesis of L-DOPA from pyrocatechol, sodium pyruvate, and ammonium acetate. The implementation of TPL for L-DOPA production has been hampered and the need for the most efficient TPL source with higher L-DOPA production and substrate conversion rate is prevailing. This study involves identifying a novel TPL from Kluyvera intermedia (Ki-TPL) and displayed a robust expression in Escherichia coli. The recombinant strain YW000 carrying Ki-TPL proved strong catalytic activity with a highest L-DOPA yield compared with 16 other TPLs from different organisms. With a further aim to improve this efficiency, random mutagenesis of Ki-TPL was performed and a mutant namely YW021 was obtained. The whole cells of YW021 as biocatalyst yielded 150.4 g L−1 of L-DOPA with a 99.99 % of pyrocatechol conversion at the optimum condition of pH 8.0 at 25 °C, which is the highest level reported to date. Further, the homology modeling and structural analysis revealed the mutant residues responsible for the extensive L-DOPA biosynthesis.





Similar content being viewed by others
References
Min, K., Park, K., Park, D. H., & Yoo, Y. J. (2015). Overview on the biotechnological production of L-DOPA. Applied Microbiology and Biotechnology, 99(2), 575–584.
Yamada, H., & Kumagai, H. (1975). Synthesis of L-tyrosine-related amino acids by beta-tyrosinase. Advances in Applied Microbiology, 19, 249–288.
Tsao, G. T., Cao, N., & Gong, C. S. (1999). Hemicellulose Conversion. In Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis and Bioseparation (Vol. 19, pp. 249–288). Wiley Inc.. ISBN: 0-471-13822-3.
Koyanagi, T., Katayama, T., Suzuki, H., Nakazawa, H., Yokozeki, K., & Kumagai, H. (2005). Effective production of 3,4-dihydroxyphenyl-L-alanine (L-DOPA) with Erwinia herbicola cells carrying a mutant transcriptional regulator TyrR. Journal of Biotechnology, 115(3), 303–306.
Tan, D., Zhao, J. P., Ran, G. Q., Zhu, X. L., Ding, Y., & Lu, X. Y. (2019). Highly efficient biocatalytic synthesis of L-DOPA using in situ immobilized Verrucomicrobium spinosum tyrosinase on polyhydroxyalkanoate nano-granules. Applied Microbiology and Biotechnology, 103(14), 5663–5678.
Min, K., Park, D. H., & Yoo, Y. J. (2010). Electroenzymatic synthesis of l-DOPA. Journal of Biotechnology, 146(1-2), 40–44.
Agarwal, P., Pareek, N., Dubey, S., Singh, J., & Singh, R. P. (2016). Aspergillus niger PA2: a novel strain for extracellular biotransformation of l-tyrosine into l-DOPA. Amino Acids, 48(5), 1253–1262.
Chávez-Béjar, M. I., Báez-Viveros, J. L., Martínez, A., Bolívar, F., & Gosset, G. (2012). Biotechnological production of l-tyrosine and derived compounds. Process Biochemistry, 47, 1017–1026.
Harir, M., Bellahcene, M., Baratto, M. C., Pollini, S., Rossolini, G. M., Trabalzini, L., Fatarella, E., & Pogni, R. (2018). Isolation and characterization of a novel tyrosinase produced by Sahara soil actinobacteria and immobilization on nylon nanofiber membranes. Journal of Biotechnology, 265, 54–64.
Min, K., & Yoo, Y. J. (2009). Amperometric detection of dopamine based on tyrosinase-SWNTs-Ppy composite electrode. Talanta, 80(2), 1007–1011.
Li, T., & Li, X. (2014). Comprehensive mass analysis for chemical processes, a case study on L-Dopa manufacture. Green Chemistry, 16, 4241–4256.
Enei, H., Matsui, H., Nakazawa, H., Okumura, S., & Yamada, H. (1973). Synthesis of L-tyrosine or 3,4-dihydroxypheny-L-alanine from L-serine and phenol or pyrocathechol. Agricultural and Biological Chemistry, 37, 493–499.
Lee, S. G., Hong, S. P., Kim, D. Y., Song, J. J., Ro, H. S., & Sung, M. H. (2006). Inactivation of tyrosine phenol-lyase by Pictet-Spengler reaction and alleviation by T15A mutation on intertwined N-terminal arm. Federation of European Biochemical Societies Journal, 273, 5564–5573.
Foor, F., Morin, N., & Bostian, K. A. (1993). Production of l-dihydroxyphenylalanine in Escherichia coli with the tyrosine phenol-lyase gene cloned from Erwinia herbicola. Applied and Environmental Microbiology, 59(9), 3070–3075.
Dennig, A., Busto, E., Kroutil, W., & Faber, K. (2015). Biocatalytic onepot synthesis of L-tyrosine derivatives from monosubstituted benzenes, pyruvate, and ammonia. American Chemical Society Catalysis, 5, 7503–7506.
Smith, H. Q., & Somerville, R. L. (1997). The tpl promoter of Citrobacter freundii is activated by the TyrR protein. Journal of Bacteriology, 179(18), 5914–5921.
Kim, D. Y., Rha, E., Choi, S. L., Song, J. J., Hong, S. P., Sung, M. H., & Lee, S. G. (2007). Development of bioreactor system for l-tyrosine synthesis using thermostable tyrosine phenol-lyase. Journal of Microbiology and Biotechnology, 17(1), 116–122.
Koyanagi, T., Katayama, T., Suzuki, H., Onishi, A., Yokozeki, K., & Kumagai, H. (2009). Hyperproduction of 3,4-Dihydroxyphenyll-alanine (l-Dopa) using Erwinia herbicola cells carrying a mutant transcriptional regulator TyrR. Bioscience, Biotechnology, and Biochemistry, 73, 1221–1223.
Chandel, M., & Azmi, W. (2013). Purification and characterization of tyrosine phenol lyase from Citrobacter freundii. Applied Biochemistry and Biotechnology, 171, 2040–2052.
Tang, X. L., Liu, X., Suo, H., Wang, Z. C., Zheng, R. C., & Zheng, Y. G. (2018). Process development for efficient biosynthesis of l-DOPA with recombinant Escherichia coli harboring tyrosine phenol lyase from Fusobacterium nucleatum. Bioprocess and Biosytems Engineering, 41, 1347–1354.
Dar, M. A., Pawar, K. D., & Pandit, R. S. (2017). Gut microbiome analysis of snails: a biotechnological approach. In S. Ray (Ed.), Organismal and Molecular Malacology. Rijeka Croatia, European Union: Intech Publishers ISBN-978-9535154075.
Chandel, M., & Azmi, W. (2009). Optimization of process parameters for the production of tyrosine phenol lyase by Citrobacter freundii MTCC 2424. Bioresource Technology, 100(5), 1840–1846.
Tang, X. L., Suo, H., Zheng, R. C., & Zheng, Y. G. (2018). An efficient colorimetric high-throughput screening method for synthetic activity of tyrosine phenol-lyase. Analytical Biochemistry, 560, 7–11.
Krissinel, E., & Henrick, K. (2005). Detection of protein assemblies in crystals. In M. R. Berthold (Ed.), Comp Life 2005, LNBI 3695, pp 163-174.
Robert, X., & Gouet, P. (2014). Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research, 42, W320–W324.
Abe, M., Iwaoka, M., Nakamura, T., Kitta, Y., Takona, H., Kodama, Y., Kawabata, K., Obata, J., Mende, A., Kobayashi, T., Fujioka, D., Saito, Y., Hasebe, H., & Kugiyama, K. (2007). Association of high level of plasma free dopamine with future coronary artery disease. Circulation Journal, 71(5), 688–692.
Lee, J. Y., & Xun, L. (1998). Novel biological process for L-DOPA production from L-tyrosine by p-hydroxyphenylacetate 3-hydroxylase. Biotechnology Letters, 20, 479–482.
Seetharam, G., & Saville, B. A. (2002). L-DOPA production from tyrosinase immobilized on zeolite. Enzyme and Microbial Technology, 31, 747–753.
Kumar, V., & Azmi, W. (2007). Microbial tyrosine phenol lyase and its application in the development of therapeutic compound. In P. S. Trivedi (Ed.), Biotechnology: Current Perspectives and Potential Applications (pp. 175–185). Rajsthan, India: Avishkar Publishers and Distributors.
Katayama, T., Suzuki, H., Koyanagi, T., & Kumagai, H. (2000). Cloning and random mutagenesis of the Erwinia herbicola tyrR gene for high-level expression of tyrosine phenollyase. Applied and Environmental Microbiology, 66(11), 4764–4771.
Zheng, R. C., Tang, X. L., Suo, H., Feng, L. L., Liu, X., Yang, J., & Zheng, Y. G. (2017). Biochemical characterization of a novel tyrosine phenol-lyase from Fusobacterium nucleatum for highly efficient biosynthesis of L-DOPA. Enzyme and Microbial Technology, 112, 88–93.
Lütke-Eversloh, T., Santos, C. N. S., & Stephanopoulos, G. (2007). Perspectives of biotechnological production of L-tyrosine and its application. Applied Microbiology and Biotechnology, 77(4), 751–762.
Wachtmeister, J., & Rother, D. (2016). Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Current Opinion in Biotechnology, 42, 169–177.
Lee, S. G., Hong, S. P., Kwak, M. S., Esaki, N., & Sung, M. H. (1999). Characterization of thermostable tyrosine phenol-lyase from an obligatory symbiotic thermophile, Symbiobacterium sp. SC-1. Journal of Biochemistry and Molecular Biology, 32, 480–485.
Kumagai, H., Kashima, N., Torii, H., Yamad, H., Enei, H., & Okumura, S. (1972). Purification, crystallization and properties of tyrosine phenol lyase from Erwinia herbicola. Agricultural and Biological Chemistry, 36, 472–482.
Sundararaju, B., Chen, H., Shilcutt, S., & Phillips, R. S. (2000). The role of glutamic acid-69 in the activation of Citrobacter freundii tyrosine phenol-lyase by monovalent cations. Biochemistry, 39(29), 8546–8555.
Milić, D., Demidkina, T. V., Faleev, N. G., Matković-Calogović, D., & Antson, A. A. (2008). Insights into the catalytic mechanism of tyrosine phenol-lyase from X-ray structures of quinonoidintermediates. Journal of Biological Chemistry, 283(43), 29206–29214.
Pelay-Gimeno, M., Glas, A., Koch, O., & Grossmann, T. N. (2015). Structure-based design of inhibitors pf protein-protein interactions: mimicking peptide binding epitopes. Angewandte Chemie International Edition in English, 54, 8896–8927.
Nagasawa, T., Utagawa, T., Goto, J., Kim, C. J., Tani, Y., Kumagai, H., & Yamada, H. (1981). Syntheses of L-tyrosine-related amino acids by tyrosine phenol-lyase of Citrobacter intermedius. European Journal of Biochemistry, 117(1), 33–40.
Chen, H. Y., Demidkina, T. V., & Phillips, R. S. (1995). Site-directed mutagenesis of tyrosine to phenylalanine in Citrobacter freundii tyrosine phenol-lyase: evidence for dual roles of tyrosine 71 as a general acid catalyst in the reaction mechanism and in cofactor binding. Biochemistry, 34(38), 12276–12283.
Nagatsua, T., & Sawadab, M. (2009). L-Dopa therapy for Parkinson’s disease: past, present, and future. Parkinsonism & Related Disorders, 15, 3–8.
Shao, Y., Arias-Cordero, E., Guo, H., Bartram, S., & Boland, W. (2014). In vivo Pyro-SIP assessing active gut microbiota of the cotton leafworm, Spodopteralittoralis. Public Library of Science One, 9, e85948.
Funding
This study was financially supported by the Natural Science Foundation of Zhejiang Province (Grant No. LQ18C010006 and LY19C010005) and National Natural Science Foundation of China (Grant Number: 31900497).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Research Involving Human Participants and/or Animals
This study is not involving any human participants and/or animals.
Informed Consent
All the authors mentioned in the manuscript have agreed for authorship, read and approved the manuscript, and given consent for submission and subsequent publication of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary file S1:
A multiple sequence alignment of homologous proteins colored according to residue conservation. (PDF 32 kb)
Rights and permissions
About this article
Cite this article
Yuan, W., Zhong, S., Xiao, Y. et al. Efficient biocatalyst of L-DOPA with Escherichia coli expressing a tyrosine phenol-lyase mutant from Kluyvera intermedia. Appl Biochem Biotechnol 190, 1187–1200 (2020). https://doi.org/10.1007/s12010-019-03164-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03164-1