Abstract
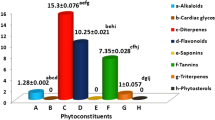
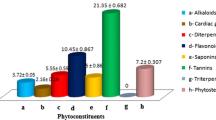
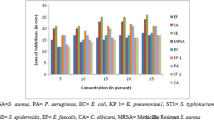
Nature is a generous source of compounds with the potential for prevention of infections. Antimicrobial screening of aqueous extract from bark of wild Himalayan cherry (Prunus cerasoides) was carried out against various pathogenic microorganisms with inhibition zone ranging from 19 to 24 mm. An optimization strategy, which included classical method and statistical method (RSM), was applied to optimize the effect of process variables. Fifteen percent plant material extracted at 40 °C for 60 min and at its natural pH (4.5) exhibited best antimicrobial activity with an average zone of inhibition ranging from 19 to 29 mm. Statistical optimization using RSM further enhanced the activity by 1.09–1.24 folds. Minimum inhibitory concentration of the aqueous extract against different microorganism ranged from 1 to 10 mg/ml. The aqueous extract was found to be reasonably thermostable at boiling temperature for 1 h. Viable cell count (VCC) studies of the extract showed it to be bactericidal in nature. Further, the aqueous extract was found to be neither cytotoxic nor mutagenic, when evaluated by MTT assay and Ames mutagenicity test. The results suggest that the aqueous extract of P. cerasoides could be a potential source to obtain new antimicrobials and effective herbal medicines to combat the problem of ever emerging microbial resistance.









Similar content being viewed by others
References
Cunha, B. A. (2001). Antibiotic side effects. Medical Clinics of North America, 85, 149–185.
Ahmad, I., & Beg, A. Z. (2001). Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology, 74, 113–123.
Dash, M., Patra, J. K., & Panda, P. P. (2008). Phytochemical and antimicrobial screening of extracts of Aquilaria agallocha Roxb. African Journal of Biotechnology, 7, 3531–3534.
Cox, P. A., & Balick, M. J. (1994). The ethnobotanical approach to drug discovery. Scientific American, 270, 60–65.
Nadkarni, K. M. (2002) Indian Materia Medica, 2nd ed., Bombay Popular Prakashan, pp. 10–16.
Pullaiah, T. (2006). Encyclopaedia of world medicinal plants, vol. 1 (p. 1615). New Delhi: Regency Publication, West Patel Nagar.
Chatterjee, A., & Pakrashi, S. C. (1992). The treatise on Indian medicinal plants. New Delhi: Publications and Information Directorate CSIR.
Dhar, M. L., Dhar, M. M., Mehrotra, D. B. N., & Ray, C. (1968). Screening of Indian plants for biological activity. Indian Journal of Experimental Biology, 6(1968), 232.
Joseph, N., Anjum, N., & Tripathi, Y. C. (2016). Phytochemical screening and evaluation of polyphenols, Flavonoids and Antioxidant Activity of Prunus cerasoides D. Don Leaves. Journal of Pharmacy Research, 10, 502–508.
Sharma, B. C. (2013). In vitro antibacterial activity of certain folk medicinal plants from Darjeeling Himalayas used to treat microbial infections. Journal of Pharmacognosy and Phytochemistry, 2, 1–4.
Bamola, N., Bisht, G., & Singh, L. (2008). In vitro antimicrobial activity of some medicinal plants of Garhwal. Plant Archives, 8, 133–137.
Arora, D. S., & Onsare, J. G. (2014). In vitro antimicrobial potential, biosafety and bioactive phytoconstituents of Moringa oleifera stem bark. World journal of pharmaceutical research, 3, 2772–2788.
Arora, D. S., Nim, L., & Kaur, H. (2016). Antimicrobial potential of Callistemon lanceolatus seed extract and its statistical optimization. Applied Biochemistry and Biotechnology, 180, 289–305.
Katapodis, P., Christakopoulou, V., Kekos, D., & Christakopoulos, P. (2007). Optimization of xylanase production by Chaetomium thermophilum in wheat straw using response surface methodology. Biochemical Engineering Journal, 35, 136–141.
Mahajan, V. (1992). Comparative evaluation of sensitivity of human pathogenic bacteria to tea, coffee and antibiotics, PhD thesis. Rohtak, India: MD University.
Parekh, J., & Chanda, S. (2006). In vitro antimicrobial activities of extracts of Launaea procumbens Roxb. (Labiateae), Vitis vinifera L. (Vitaceae) and Cyperus rotundus L. (Cyperaceae). African. Journal of biomedical Research, 9, 8993.
Suzuki, H., Okubo, L., Yamazaki, S., Suzuki, K., Mitsuya, H., & Toda, S. (1989). Inhibition of the infectivity and cytopathic effect of the human immunodeficiency virus by water soluble lignin in an extract of the culture medium of Lentinus edodes mycelia (LEM). Biochemical and Biophysical Research Communications, 60, 367–373.
Mortelmans, K., & Zeiger, E. (2000). The Ames Salmonella/microsome mutagenicity assay. Mutation Research, 455, 29–60.
Kaur, H., Arora, D. S., & Sharma, V. (2014). Isolation, purification, and characterization of antimicrobial compound 6-[1,2-dimethyl-6-(2-methyl-allyloxy)-hexyl]-3-(2-methoxy-phenyl)-chromen-4-one from Penicillium sp. HT-28. Applied Biochemistry and Biotechnology, 173, 1963–1976.
Ong, T., Whong, W. Z., Stewart, J. D., & Brockman, H. E. (1986). Chlorophyllin: a potent antimutagen against environmental and dietary complex mixtures. Mutation Research Letters, 173, 111–115.
Negi, P., Jayaprakasha, G., & Jena, B. S. (2003). Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chemistry, 80, 393–397.
Janssen, A. M., Scheffer, J. J. C., & Baerheim, S. A. (1976). Antimicrobial activity of essential oils. Planta Medica, 53, 395–398.
Newman, D. J., & Cragg, G. M. (2007). Natural products as source of new drugs over the last 25 years. Journal of Natural Products, 70, 461–477.
Muthu, M., Gopal, J., Min, S. X., & Chun, S. (2016). Green tea versus traditional Korean teas: antibacterial/antifungal or both? Applied Biochemistry and Biotechnology, 180, 780–790.
Onsare, J. G., Kaur, H., & Arora, D. S. (2013). Antimicrobial activity of Moringa oleifera from different locations against some human pathogens. Academia Journal of Medicinal Plants, 1, 080–091.
Arora, D.S., & Sood, H. (2017). In vitro antimicrobial potential of extracts and phytoconstituents from Gymnema sylvestre R.Br. leaves and their biosafety evaluation. AMB Express, 7, 115. https://doi.org/10.1186/s13568-017-0416-z.
Abubakar, E. M. (2010). Antibacterial potential of crude leaf extracts of Eucalyptus camaldulensis against some pathogenic bacteria. African Journal of Plant Science, 4, 202–209.
Kaur, G. J., & Arora, D. S. (2009). Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complementary and Alternative Medicine, 9, 30.
Adeshina, G., Okeke, C., Onwuegbuchulam, N., & Ehinmidu, J. (2009). Preliminary studies on antimicrobial activities of ethanolic extracts of Ficus sycomorus Linn. and Ficus platyphylla Del. (Moraceae). International Journal of Biological and Chemical Sciences, 3, 147–151.
Thially, B. G., Milena, A. B., Francisco, F. M., Gilvandete, M. P., Cibele, B. M., Paula, B., Thiago, M., Jeanlex, S. S., Eduardo, B. B., Ronaldo, F., & Aparecida, T. (2012). Effect of subinihibitory and inhibitory concentrations of Plectranthus amboinicus (Lour.) Spreng essential oil on Klebsiella pneumoniae. Phytomedicine, 19, 962–968.
Wu, Q. L., Chen, T., Gan, Y., Chen, X., & Zhao, X. M. (2007). Optimization of riboflavin production by recombinant Bacillus subtilis RH44 using statistical designs. Applied Microbiology and Biotechnology, 76, 783–794.
Maher, O., Moharnad, S., Moharmnad, A., Enas, A., & Hanee, A. (2012). Antimicrobial activity of crude extracts of some plant leaves. Research Journal of Microbiology, 7, 59–67.
Adeyemi, I. A., Christiana, I. A., Linda, N. E., Adebiyi, A., & Emmanuel, A. O. (2009). Antimicrobial and toxicological studies of epa-ijebu. a “wonder—Cure” concoction used in south-west, Nigeria. African Journal of Infectious Diseases, 3, 6–13.
Regasini, L. O., Cotinguiba, F., Passerini, G. D., Bolzani, V. S., Cicarelli, R. M. B., Kato, M. J., & Furlan, M. (2009). Trypanocidal activity of Piper arboreum and Piper tuberculatum (Piperaceae). Revista Brasileira de Farmacognosia, 19, 199–203.
Helmerhorst, E. J., Reijnders, I. M., Hof, W. V. T., Smit, I. S., Veerman, E. C. J., & Amerongen, A. V. N. (1999). Amphotericin B and fluconazole-resistant Candida spp, Aspergillus fumigatus and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrobial Agents and Chemotherapy, 43, 702–704.
Masoko, P., Mmushi, T. J., Mogashoa, M. M., Mokgotho, M. P., Mampuru, L. J., & Howard, R. L. (2008). In vitro evaluation of the antifungal activity of Sclerocarya birrea extracts against pathogenic yeasts. African Journal of Biotechnology, 7, 3521–3526.
Acknowledgements
The support offered to Himadri in the form of fellowship under university with potential for excellence (UPE) scheme of the UGC New Delhi assisted to the university.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(TIFF 302 kb)
Rights and permissions
About this article
Cite this article
Arora, D.S., Mahajan, H. In Vitro Evaluation and Statistical Optimization of Antimicrobial Activity of Prunus cerasoides Stem Bark. Appl Biochem Biotechnol 184, 821–837 (2018). https://doi.org/10.1007/s12010-017-2571-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2571-8