Abstract
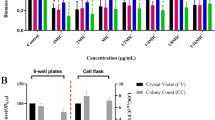
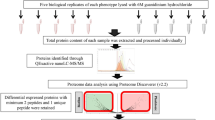
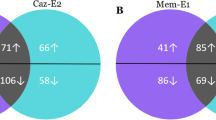
Pseudomonas aeruginosa biofilm-related infections are the major cause of premature death in cystic fibrosis patients. Strategies to induce biofilm dispersal are of interest, because of their potential in preventing biofilm-related infections. Our previous work demonstrated that n-butanolic Cyclamen coum extract with ciprofloxacin could eliminate 1- and 3-day-old P. aeruginosa PAO1 biofilms. To gain new insights into the role of C. coum extract and its synergistic effect with ciprofloxacin in eliminating P. aeruginosa PAO1 biofilms, two-dimensional gel electrophoresis (2-DE) in combination with mass spectrometry-based protein identification were used. Changes in the bacterial protein expression were analyzed when 3-day-old biofilm cells were exposed to the C. coum extract alone and in combination with ciprofloxacin. Proteins involved in alginate biosynthesis, quorum sensing, adaptation/protection, carbohydrate and amino acid metabolism showed a weaker expression in the C. coum extract-ciprofloxacin-treated biofilm cells compared to those in the untreated cells. Interestingly, the proteome of C. coum extract-ciprofloxacin-treated biofilm revealed more resemblance to the planktonic phenotype than to the biofilm phenotype. It appears that saponin extract in combination with ciprofloxacin causes biofilm disruption due to several mechanisms such as motility induction, cell envelope integrity perturbation, stress protein expression reduction, and more importantly, signal transduction perturbation. In conclusion, exposure to a combination of biofilm dispersal such as saponin extract and antimicrobial agents may offer a novel strategy to control preestablished, persistent P. aeruginosa biofilms and biofilm-related infections.


Similar content being viewed by others
Abbreviations
- 2-DE:
-
Two-dimensional gel electrophoresis
- TSA:
-
Tryptic soy agar
- TSB:
-
Trypticase soy broth
- IPG:
-
Immobilized pH gradient
- PMSF:
-
Ammonium persulfate and phenylmethylsulfonylfluoride
- THU:
-
Tehran Herbarium University
- DMSO:
-
Dimethyl-sulphoxide
- FBECs:
-
Fractional biofilm eliminating concentration
- MBEC:
-
Minimum biofilm eliminating concentration
- BSA:
-
Bovine serum albumin
- ACN:
-
Acetonitrile
- CAM:
-
Carbamidomethyl
- Me:
-
Methionine
- FA:
-
Formic acid
- SD:
-
Standard deviations
- Fgt:
-
Flagellar glycosyl transferase
- Ndk:
-
Nucleoside diphosphate kinase
- AHL:
-
Acyl-homoserine lactone
References
Høiby, N., Ciofu, O., & Bjarnsholt, T. (2010). Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiology, 5, 1663–1674.
Davies, D. (2003). Understanding biofilm resistance to antibacterial agents. Nature Reviews of Drug Discovery, 2, 114–122.
Kaplan, J. B. (2010). Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. Journal of Dental Research, 89, 205–218.
Nyakudya, E., Jeong, J. H., Lee, N. K., & Jeong, Y. S. (2014). Platycosides from the roots of Platycodon grandiflorum and their health benefits. Preventive Nutrition and Food Science, 19, 59–68.
Shafiei, M., Abdi-Ali, A., Shahcheraghi, F., Saboora, A., & Akbari-Noghabi, K. (2014). Eradication of Pseudomonas aeruginosa biofilms using the combination of n-butanolic Cyclamen coum extract and ciprofloxacin. Jundishapur Journal of Microbiology, 7, e17430.
Ahmadbeigi, Z., & Saboora, A. (2009). Comparison of three methods for saponin extraction from tuber of Cyclamen coum Miller. Journal of Science, 21, 27–38.
Hassan, S. M., Al- Aqil, A. A., & Attimarad, M. (2013). Determination of crude saponin and total flavonoids content in guar meal. Advancement in Medicinal Plant Research, 1, 24–28.
Pennsylvania, W. (2007). Clinical and Laboratory Standards Institute, CLSI (Clinical and Laboratory Standards Institute). Performance standards for antimicrobial susceptibility testing, approved standard M100-S17. 17th Informational Supplement.
Pitts, B., Hamilton, M. A., Zelver, N., & Stewart, P. S. (2003). A microtiter plate screening method for biofilm disinfection and removal. Journal of Microbiological Methods, 54, 269–276.
Oosthuizen, M. C., Steyn, B., Theron, J., Cosette, P., Lindsay, D., Holy, A. V., & Brozel, V. S. (2002). Proteomic analysis reveals differential protein expression by Bacillus cereus during biofilm formation. Applied and Environmental Microbiology, 68, 2770–2780.
Molloy, M.P., Herbert, B.R., Walsh, B.J., Tyler, M.I., Traini, M., Sanchez, J.C., Hochstrasser, D.F., Williams, K.L., Gooley., A.A. (1998). Extraction of membrane proteins by differential solubilization for separation using two- dimensional gel electrophoresis. Electrophoresis, 19(5), 837–44.
Blum, H., Beier, H., & Gross, H. J. (1987). Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis, 8, 93–99.
Masoudzadeh, N., Alidoust, L., Samie, N., Hajfarajollaha, H., Sharafi, H., Modiria, S., Zahiri, S. H., Vali, H., & Noghabi, K. A. (2014). Distinctive protein expression patterns of the strain Brevundimonas sp. ZF12 isolated from the aqueous zone containing high levels of radiation to cadmium-induced stress. Journal of Biotechnology, 186, 49–57.
Vyshnava, S. S., Kanderi, D. P., Panjala, S. P., Pandian, P., Bontha, R. R., Goukanapalle, P. K. R., & Banaganapalli, B. (2016). Effect of silver nanoparticles against the formation of biofilm by Pseudomonas aeruginosa an in silico approach. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-016-2107-7.
Izano, E., Amarante, M., Kher, W., & Kaplan, J. (2008). Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Applied and Environmental Microbiology, 74, 470–476.
Goldberg, J., Hatano, K., & Pier, G. B. (1993). Synthesis of lipopolysaccharide-O-side-chains by Pseudomonas aeruginosa PAO1 requires the enzyme phosphomannomutase. Journal of Bacteriology, 175, 1605–1611.
De Vos, D., Jr, A. L., Pirnay, J. P., Struelens, M., Vandenvelde, C., Duinslaeger, L., Vanderkelen, A., & Cornelis, P. (1997). Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. Journal of Clinical Microbiology, 35, 1295–1299.
Sauer, K., Camper, A., Ehrlich, G., & Costerton, J. D. (2002). Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. Journal of Bacteriology, 184, 1140–1154.
Stoodley, P., Hall-Stoodley, L., Boyle, J. D., Lappin-Scott, H. M., & Costerton, J. W. (2001). Growth and detachment of cell clusters from mature mixed-species biofilms. Applied and Environmental Microbiology, 67, 5608–5613.
Clarke, M., Hughes, D., Zhu, C., Boedeker, E., & Sperandio, V. (2006). The QseC sensor kinase: a bacterial adrenergic receptor. Proceedings of the National Academy of Sciences, 103, 10420–10425.
Acknowledgments
The corresponding author would like to thank the National Institute of Genetic Engineering and Biotechnology (NIGEB) and the Vice Chancellor of the Alzahra University for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shafiei, M., Abdi-Ali, A., Shahcheraghi, F. et al. Analysis of Pseudomonas aeruginosa PAO1 Biofilm Protein Profile After Exposure to n-Butanolic Cyclamen coum Extract Alone and in Combination with Ciprofloxacin. Appl Biochem Biotechnol 182, 1444–1457 (2017). https://doi.org/10.1007/s12010-017-2409-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2409-4