Abstract
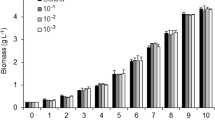
Photosynthetic mitigation of CO2 through microalgae is gaining great importance due to its higher photosynthetic ability compared to plants, and the biomass can be commercially exploited for various applications. CO2 fixation capability of the newly isolated freshwater microalgae Scenedesmus bajacalifornicus BBKLP-07 was investigated using a 1-l photobioreactor. The cultivation was carried at varying concentration of CO2 ranging from 5 to 25%, and the temperature and light intensities were kept constant. A maximum CO2 fixation rate was observed at 15% CO2 concentration. Characteristic growth parameters such as biomass productivity, specific growth rate, and maximum biomass yield, and biochemical parameters such as carbohydrate, protein, lipid, chlorophyll, and carotenoid were determined and discussed. It was observed that the effect of CO2 concentration on growth and biochemical composition was quite significant. The maximum biomass productivity was 0.061 ± 0.0007 g/l/day, and the rate of CO2 fixation was 0.12 ± 0.002 g/l/day at 15% CO2 concentration. The carbohydrate and lipid content were maximum at 25% CO2 with 26.19 and 25.81% dry cell weight whereas protein, chlorophyll, and carotenoid contents were 32.89% dry cell weight, 25.07 μg/ml and 6.15 μg/ml respectively at 15% CO2 concentration.






Similar content being viewed by others
References
Cheah, W. Y., Show, P. L., Chang, J. S., Ling, T. C., & Juan, J. C. (2015). Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresource Technology., 184, 190–201.
Ramaraj, R., Tsai, D. D. W., & Chen, P. H. (2014). Freshwater microalgae niche of air carbon dioxide mitigation. Ecoloogical Engineering, 68, 47–52.
Ritschard, R. L. (1992). Marine algae as a CO2 sink. Water Air Soil Pollution, 64, 289–303.
Toledo, C. A., Morales, M., Novelo, E., & Revah, S. (2013). Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Bioresourorce Technology., 130, 652–658.
Online WMO statement on the status of the global climate in 2012. Available http://www.wmo.int/pages/prog/wcp/wcdmp/documents/WMO_1108.pdf.
Richard, S. J. T. (2009). The economic effects of climate change. Journal of Economic Perspectives, 23(2), 29–51 Springer.
Stewart, C., & Hessami, M. A. (2005). A study of methods of carbon dioxide capture and sequestration the sustainability of a photosynthetic bioreactor approach. Energy Conversion and Management., 46, 403–420.
Lara-Gil, J. A., Álvarez, M. M., & Pacheco, A. (2014). Toxicity of flue gas components from cement plants in microalgae CO2 mitigation systems. Journal of Applied Phycology., 26, 357–368.
Houser, J. B., Venable, M. E., Sakamachi, Y., Hambourger, M. S., Herrin, J., & Tuberty, S. R. (2014). Wastewater remediation using algae grown on a substrate for biomass and biofuel production. Journal of Environmental Protection., 5, 895–904.
Kightlinger, W., Chen, K., Pourmir, A., Crunkleton, D. W., Price, G. L., & Johannes, T. W. (2014). Production and characterization of algae extract from Chlamydomonas reinhardtii. Electronic Journal of Biotechnology, 17(1), 14–18.
Ho, S. H., Chen, C. Y., & Chang, J. S. (2012). Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresource Technology, 113, 244–252.
Miranda, J. R., Passarinho, P. C., & Gouveia, L. (2012). Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresource Technology, 104, 342–348.
Chiu, S. Y., Kao, C. Y., Tsai, M. T., Ong, S. C., Chen, C. H., & Lin, C. S. (2009). Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresource Technology., 100, 833–838.
de Morais, M. G., & Costa, J. A. (2007). Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Conversion and Managememnt., 48, 2169–2173.
Samarpita, B., Abhijit Sarma, R., Kaustubha, M., & Aloke, K. G. (2013). Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam, India. Bioresource Technology, 143, 369–377.
Ota, M., Kato, Y., Watanabe, H., Watanabe, M., Sato, Y., Smith, R. L., & Inomata, H. (2009). Fatty acid production from a highly CO2 tolerant alga, Chlorocuccum littorale, in the presence of inorganic carbon and nitrate. Bioresource Technology., 100, 5237–15242.
Chiu, S. Y., Kao, C. Y., Chen, C. H., Kuan, T. C., Ong, S. C., & Lin, C. S. (2008). Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresource Technology., 99, 3389–3396.
Sydney, E. B., Sturm, W., de Carvalho, J. C., Thomaz, S. V., Larroche, C., Pandey, A., & Soccol, C. R. (2010). Potential carbon dioxide fixation by industrially important microalgae. Bioresource Technology., 101, 5892–5896.
Vidyashankar, S., Deviprasad, K., Chauhan, V. S., Ravishankar, G. A., & Sarada, R. (2013). Selection and evaluation of CO2 tolerant indigenous microalga Scenedesmus dimorphus for unsaturated fatty acid rich lipid production under different culture conditions. Bioresource Technology., 144, 28–37.
Rodolfi, L., Zittelli, G. C., Bassi, N., Padovani, G., Biondi, N., Bonini, G., & Tredici, M. R. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnology and Bioengineering., 102, 100–112.
Dereeper, A., Guignon, V., Blanc, G., Audic, S., Buffet, S., Chevenet, F., Dufayard, J. F., Guindon, S., Lefort, V., Lescot, M., Claverie, J. M., & Gascuel, O. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research, 1, 36.
Mariana, A., Fernandes, B. D., Vicente, A. A., Teixeira, J. A., & Dragone, G. (2013). Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresource Technology., 139, 149–154.
Abreu, A. P., Fernandes, B., Vicente, A. A., Teixeira, J., & Dragone, G. (2012). Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresource Technology., 118, 61–66.
Tang, D., Han, W., Li, P., Miao, X., & Zhong, J. (2011). CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresource Technology., 102, 3071–3076.
Fernandes, B., Dragone, G., Abreu, A., Geada, P., Teixeira, J., Vicente, A. (2016). Starch determination in Chlorella vulgaris—a comparison between acid and enzymatic methods. Journal of Applied Phycology, 1–6.
Folch, J., Lees, M., & Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry., 226, 497–509.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry., 193, 265–275.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In L. Packer & R. Douce (Eds.), Methods in enzymology (Vol. 148, pp. 350–382). London: Academic Press.
Banerjee, A., Sharma, R., Chisti, Y., & Banerjee, U. C. (2002). Botryococcus braunii: a renewable source of hydrocarbons and other chemicals. Critical Reviews in Biotechnology., 22, 245–279.
Shakeel, A.A., Shivasharana, C.T., Kaliwal, B.B. (2016). Identification and characterisation of Chlorella vulgaris for biodiesel production. International Journal of Scientific Research and Engineering, 3(1).
Metting, B., & Pyne, J. W. (1986). Biologically-active compounds from microalgae. Enzyme and Microbial Technology., 8, 386–394.
Francisco, X., Malcata, A., Guedes, C., & Amaro, H. M. (2011). Microalgae as sources of carotenoids. Marine Drugs., 9, 625–644.
Online: “Environmental Analysis Study” (2006). Department of Rural Development and Panchayat Raj. Government of Karnataka. July 2001. https://en.wikipedia.org/wiki/Bagalkot_district#cite_note-worldbank-10.
Flechtner, V. R., Johansen, J. R., & And Clark, W. C. (1998). Algal composition of microbiotic crusts from the central desert of Baja California, Mexico. Great Basin Naturalist, 58, 295–311.
Mary, S., Sudhakar, R., Anwar, A. F., Selvaraju, G. D., & Mohandass, R. (2014). Agrobacterium-mediated transformation of three freshwater microalgal strains. Polish Journal of Microbiology., 63(4), 387–392.
Deng, D., & Tam, N. F. (2015). Isolation of microalgae tolerant to polybrominated diphenyl ethers (PBDEs) from wastewater treatment plants and their removal ability. Bioresource Technology., 177, 289–297.
Riebesell, U., Wolf-Gladrow, D. A., & Smetacek, V. S. (1993). Carbon dioxide limitation of marine phytoplankton growth rates. Nature, 361, 249–251.
Morais, M. G. D., & Costa, J. A. V. (2007). Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. Journal of Biotechnology., 129, 439–445.
Burkhardt, S., Riebesell, U., & Zondervan, I. (1999). Stable carbon isotope fractionation by marine phytoplankton in response to day length, growth rate, and CO2 availability. Marine Ecology Progress Series., 194, 31–41.
Yang, Y., & Gao, K. (2003). Effects of CO2 concentrations on the freshwater microalgae, Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (chlorophyta). Journal of Applied Phycology, 15, 1–11.
Lee, S. J., Yoon, B. D., & Oh, H. M. (1998). Rapid method for the determination of lipid from the green algae Botryococcus braunii. Biotechnology, 12(7), 553–556.
Sarat, T. C., Deepak, R. S., Maneesh, M. K., Mukherji, S., Chauhan, V. S., Sarada, R., & Mudliar, S. N. (2016). Evaluation of indigenous fresh water microalga Scenedesmus obtusus for feed and fuel applications: effect of carbon dioxide, light and nutrient sources on growth and biochemical characteristics. Bioresource Technology, 207, 430–439.
Varshney, P., Sohoni, S., Wangikar, P. P., & Beardall, J. Effect of high CO2 concentrations on the growth and macromolecular composition of a heat- and high-light-tolerant microalga. Jorrnal of Applied Phycology. doi:10.1007/s10811-016-0797-4.
Basu, S., Roy, A. S., Mohanty, K., & Ghoshal, A. K. (2013). Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam. India. Bioresource Technology., 143, 369–377.
Domozych, D. S., Ciancia, M., Fangel, J. U., Mikkelsen, M. D., Ulvskov, P., & Willats, W. G. (2012). The cell walls of green algae: a journey through evolution and diversity. Frontier Plant Science, 3, 1–7.
Moraes, L., da Rosa, G. M., Cardias, B. B., dos Santos, L. O., & Costa, J. A. V. (2016). Microalgal biotechnology for greenhouse gas control: carbon dioxidefixation by spirulina sp. at different diffusers. Ecological Engineering., 91, 426–431.
Dragone, G., Fernandes, B. D., Abreu, A. P., Vicente, A. A., & Teixeira, J. A. (2011). Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Applied Energy, 88, 3331–3335.
Šoštaricˇ, M., Klinar, D., Bricelj, M., Golob, J., Berovicˇ, M., & Likozar, B. (2012). Growth, lipid extraction and thermal degradation of the microalga Chlorella vulgaris. New Biotechnology., 29, 325–331.
Acknowledgements
The authors are profusely thankful to the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, New Delhi, for funding the Bioinformatics Infrastructure Facility Project (BT/BI/25/001/2006 VOL II date 05-03-2012) and also the Interdisciplinary Program for Life Science Project (BT/PR/4555/INF/22/126/2010 dated 30-09-2010) and the P. G Departments of Biotechnology and Microbiology, Karnatak University, Dharwad, for providing the facilities for pursuing the research work at the department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies related to animals and human participants.
Rights and permissions
About this article
Cite this article
Patil, L., Kaliwal, B. Effect of CO2 Concentration on Growth and Biochemical Composition of Newly Isolated Indigenous Microalga Scenedesmus bajacalifornicus BBKLP-07. Appl Biochem Biotechnol 182, 335–348 (2017). https://doi.org/10.1007/s12010-016-2330-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2330-2