Abstract
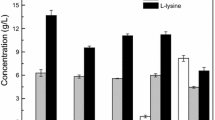
Reducing the viscosity of molasses environmentally and selectively removing the harmful ingredients for microbes are the keys to promoting the bioavailability of molasses. A simple and environmental in situ pretreatment method integrating surfactants and alkali was developed to reduce the viscosity of molasses prior to l-lysine production using Escherichia coli ZY0217. Adding activated carbon and modified orange peel based on the in situ pretreatment process effectively removed pigments and excessive zinc in the molasses and also significantly increased the cell growth and l-lysine yield from E. coli ZY0217. The experimental results showed that a mixture of secondary alkane sulfonate, an anionic surfactant, and HodagCB-6, a non-ionic surfactant, effectively reduced the viscosity of the molasses more so than any single surfactant. When the surfactant mixture was added at a concentration of 0.04 g/L to the molasses, the ω value was 0.4, and when ammonia was added at 0.6 %, the lowest viscosity of 705 mPa · s was obtained. Further, 91.5 % of the color and 86.68 % of the original levels of zinc were removed using an activated carbon and modified orange peel treatment on the molasses with the lowest viscosity, which further promoted cell growth and l-lysine production. In the fed-batch cultivation process, the l-lysine concentration achieved using a constant-speed feeding strategy was 45.89 g/L, with an l-lysine yield of 27.18 %, whereas the l-lysine yield from untreated molasses was only 10.13 %. The increase in l-lysine yield was related to the reduced viscosity and the detoxification of the molasses. Lastly, the pretreatment was found to significantly enhance the conversion of sugars in the molasses to l-lysine.



Similar content being viewed by others
References
He, X., Chen, K. Q., Li, Y., Wang, Z., Zhang, H., Qian, J., & Ouyang, P. K. (2015). Enhanced L-lysine production from pretreated beet molasses by engineered Escherichia coli in fed-batch fermentation. Bioprocess and Biosystems Engineering, 38, 1615–1622.
Xu, K., & Xu, P. (2014). Efficient production of L-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresource Technology, 153, 23–29.
Chan, S., Kanchanatawee, S., & Jantama, K. (2012). Production of succinic acid from sucrose and sugarcane molasses by metabolically engineered Escherichia coli. Bioresource Technology, 103, 329–336.
Park, S. C., & Baratti, J. (1991). Batch fermentation kinetics of sugar beet molasses by Zymomonas mobilis. Biotechnology and Bioengineering, 38, 304–313.
Abadias, M., Teixido, N., Usall, J., Vinas, I., & Magan, N. (2000). Solute stresses affect growth patterns, endogenous water potentials and accumulation of sugars and sugar alcohols in cells of the biocontrol yeast Candida sake. Journal of Applied Microbiology, 89, 1009–1017.
Paula, F. S., Susan, G. K., Júlio, C. C., Wilerson, S., José, A. R. L., Jean, L. T., Reeta, R. S., Ashok, P., & Carlos, R. S. (2008). Production of bio-ethanol from soybean molasses by Saccharomyces cerevisiae at laboratory, pilot and industrial scales. Bioresource Technology, 99, 8156–8163.
Togrul, H., & Arslan, N. (2004). Mathematical model for prediction of apparent viscosity of molasses. Journal of Food Engineering, 62, 281–289.
Kaur, S., Kaler, R. S. S., & Aamarpali, A. (2002). Effect of starch on the rheology of molasses. Journal of Food Engineering, 55, 319–322.
Roukas, T. (1998). Pretreatment of beet molasses to increase pullulan production. Process Biochemistry, 33, 805–810.
Faruk, K., Hande, K., Dilvin, G., Ilaria, F., Annarita, P., Orhan, Y., Barbara, N., & Ebru, T. Ö. (2011). Molasses as fermentation substrate for levan production by Halomonas sp. Applied Microbiology and Biotechnology, 89, 1729–1740.
Marty, R. J., & Demeyer, D. I. (1973). The effect of inhibitors of methane production on fermentation pattern and stoichiometry in vitro using rumen contents from sheep given molasses. The British Journal of Nutrition, 30, 369–376.
Chen, K. Q., He, X., Zhang, H., Qi, Y. B., & Ouyang, P. K. A novel composite viscosity reducer and its application in the reducing the molasses viscosity. China Patent No. 201510773321.X.
Nguyen, T. A. H., Ngo, H. H., Guo, W. S., Zhang, J., Liang, S., Yue, Q. Y., Li, Q., & Nguyen, T. V. (2013). Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresource Technology, 148, 574–585.
Ying, H. X., He, X., Li, Y., Chen, K. Q., & Ouyang, P. K. (2014). Optimization of culture conditions for enhanced lysine production using engineered Escherichia coli. Applied Biochemistry and Biotechnology, 172, 3835–3843.
Saurina, J., Hernandez-Cassou, S., Alegret, S., & Fabregas, E. (1999). Amperometric determination of lysine using a lysine oxidase biosensor based on rigid-conducting composites. Biosensors and Bioelectronics, 14, 211–220.
Dubois, M. A., Gilles, K. A., Hamilton, J. K., Robers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Liu, Q., Dong, M. Z., Ma, S. Z., & Tu, Y. (2007). Surfactant enhanced alkaline flooding for Western Canadian heavy oil recovery. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 293, 63–71.
Zengin, H., & Erkan, B. (2009). Adsorption of acids and bases from aqueous solutions onto silicon dioxide particles. Journal of Hazardous Materials, 172, 978–985.
Nitschke, M., & Costa, S. G. (2007). Biosurfactants in food industry. Trends in Food Science & Technology, 18, 252–259.
Garti, N., Clement, V., Fanun, M., & Leser, M. E. (2000). Some characteristics of sugar ester nonionic microemulsions in view of possible food applications. Journal of Agricultural and Food Chemistry, 48, 3945–3956.
Chortyk, O. T., Kays, S. J., & Teng, Q. (1997). Characterization of insecticidal sugar esters of petunia. Journal of Agricultural and Food Chemistry, 45, 270–275.
Claudia, B., Andres, I., & Lorena, W. (2015). Improvement of efficiency in the enzymatic synthesis of Lactulose Palmitate. Journal of Agricultural and Food Chemistry, 63, 3716–3724.
Song, D. D., Li, Y. M., Liang, S. K., & Wang, J. T. (2013). Micelle behaviors of sophorolipid/rhamnolipid binary mixed biosurfactant systems. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 436, 201–206.
Ahmadi, M., Vahabzadeh, F., Bonakdarpour, B., & Mehranian, M. (2006). Empirical modeling of olive oil mill wastewater treatment using loofa-immobilized Phanerochaete chrysosporium. Process Biochemistry, 41, 1148–1154.
Gilston, B. A., Wang, S. N., Marcus, M. D., Canalizo-Hernández, M. A., Swindell, E. P., Xue, Y., Mondragón, A., & O’Halloran, T. V. (2014). Structural and mechanistic basis of zinc regulation across the E. coli Zur Regulon. PLOS Biology, 12, e1001987.
Nies, D. H. (1999). Microbial heavy-metal resistance. Applied Microbiology and Biotechnology, 51, 730–750.
Takahashi, H., Oshima, T., Hobman, J. L., Doherty, N., Clayton, S. R., Iqbal, M., Hill, P. J., Tobe, T., Ogasawara, N., Kanaya, S., & Stekel, D. J. (2015). The dynamic balance of import and export of zinc in Escherichia coli suggests a heterogeneous population response to stress. Journal of the Royal Society Interface, 12, 20150069.
Acknowledgments
This work was supported by the “863” program of China (Grant Nos. 2015AA021005, 2014AA021703) and Guangxi Science and Technology Development Program (Grant No. 1598004-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
He, X., Qi, Y., Chen, K. et al. Enhancing l-Lysine Production of Beet Molasses by Engineered Escherichia coli Using an In Situ Pretreatment Method. Appl Biochem Biotechnol 179, 986–996 (2016). https://doi.org/10.1007/s12010-016-2045-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2045-4