Abstract
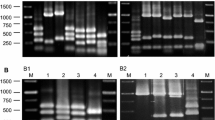
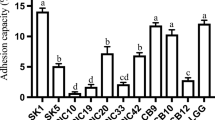
Lactic acid bacteria (LAB, n = 57) were previously obtained from raw goat milk, identified as Lactococcus spp. (n = 24) and Enterococcus spp. (n = 33), and characterized as bacteriocinogenic. Fingerprinting by pulsed field gel electrophoresis (PFGE) demonstrated high genetic diversity, and 30 strains were selected and exhibited strong antimicrobial activity against 46 target strains (LAB, spoilage, and foodborne pathogens). Six strains (Lactococcus lactis: GLc03 and GLc05; and Enterococcus durans: GEn09, GEn12, GEn14, and GEn17) were selected to characterize their bacteriocinogenic features, using Listeria monocytogenes ATCC 7644 as the target. The six strains produced bacteriocins at higher titer when incubated in MRS at 37 °C up to 12 h, when compared to growth at 25 and 30 °C. The produced bacteriocins kept their antimicrobial activity after exposure to 100 °C for 2 h and 121 °C for 20 min; the antimicrobial activity was also observed after treatment at pH 2.0 to 10.0, except for GLc03. L. monocytogenes populations were reduced approximately two logs after treatment with cell-free supernatants from the selected strains. These data show that goat milk can contain a diverse microbiota able to inhibit L. monocytogenes, a common pathogen found in dairy products, and can be potentially employed in biopreservation of food produced under different processing conditions.




Similar content being viewed by others
References
Aasen, I. M., Moretro, T., Katla, T., Axelsson, L., & Storro, I. (2000). Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl Microbiol Biotechnol, 53, 159–166.
Achemchem, F., Cebrian, R., Abrini, J., Martinez-Bueno, M., Valdivia, E., & Maqueda, M. (2012). Antimicrobial characterization and safety aspects of the bacteriocinogenic Enterococcus hirae F420 isolated from Moroccan raw goat milk. Can J Microbiol, 58, 596–604.
Achemchem, F., Martinez-Bueno, M., Abrini, J., Valdivia, E., & Maqueda, M. (2005). Enterococcus faecium F58, a bacteriocinogenic strain naturally occurring in Jben, a soft, farmhouse goat's cheese made in Morocco. J Appl Microbiol, 99, 141–150.
Ahmadova, A., Todorov, S. D., Choiset, Y., Rabesona, H., Tannaz, M. Z., Kuliyev, A., Franco, B. D. G. M., Chobert, J. M., & Haertlé, T. (2013). Evaluation of antimicrobial activity, probiotic properties and safety of wild strain Enterococcus faecium AQ71 isolated from Azerbaijani Motal cheese. Food Control, 30, 631–641.
Ananou, S., Maqueda, M., Martinez-Bueno, M., Galvez, A., & Valdivia, E. (2005). Control of Staphylococcus aureus in sausages by enterocin AS-48. Meat Sci, 71, 549–556.
Badis, A., Guetarni, D., Moussa-Boudjemâa, B., Henni, D. E., Tornadijo, M. E., & Kihal, M. (2004). Identification of cultivable lactic acid bacteria isolated from Algerian raw goat’s milk and evaluation of their technological properties. Food Microbiol, 21, 343–349.
Barros, M. A. F., Nero, L. A., Silva, L. C., D’Ovidio, L., Monteiro, F. A., Tamanini, R., Fagnani, R., Hofer, E., & Beloti, V. (2007). Listeria monocytogenes: occurrence in beef and identification of the main contamination points in processing plants. Meat Sci, 76, 591–596.
Batdorj, B., Dalgalarrondo, M., Choiset, Y., Pedroche, J., Metro, F., Prevost, H., Chobert, J. M., & Haertle, T. (2006). Purification and characterization of two bacteriocins produced by lactic acid bacteria isolated from Mongolian airag. J Appl Microbiol, 101, 837–848.
Biscola, V., Todorov, S. D., Capuano, V. S., Abriouel, H., Galvez, A., & Franco, B. D. (2013). Isolation and characterization of a nisin-like bacteriocin produced by a Lactococcus lactis strain isolated from charqui, a Brazilian fermented, salted and dried meat product. Meat Sci, 93, 607–613.
Bromberg, R., Moreno, I., Delboni, R. R., & Cintra, H. C. (2006). Características da bacteriocina produzida por Lactococcus lactis subsp. hordniae CTC 484 e seu efeito sobre Listeria monocytogenes em carne bovina. Food Sci. Technol, 26, 135–144.
Campos, C. A., Rodriguez, O., Calo-Meta, P., Prado, M., & Barros-Velazquez, J. (2006). Preliminary characterization of bacteriocins from Lactococcus lactis, Enterococcus faecium and Enterococcus mundtii strains isolated from turbot (Psetta maxima). Food Res Int, 39, 356–364.
Castro, M. P., Palavecino, N. Z., Herman, C., Garro, O. A., & Campos, C. A. (2011). Lactic acid bacteria isolated from artisanal dry sausages: characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Sci, 87, 321–329.
Centeno, J. A., Fernandez-Garcia, E., Gaya, P., Tomillo, J., Medina, M., & Nunez, M. (2004). Volatile compounds in cheeses made from raw ewes’ milk ripened with a lactic culture. J Dairy Res, 71, 380–384.
Cocolin, L., Foschino, R., Comi, G., & Grazia Fortina, M. (2007). Description of the bacteriocins produced by two strains of Enterococcus faecium isolated from Italian goat milk. Food Microbiol, 24, 752–758.
Cosentino, S., Fadda, M. E., Deplano, M., Melis, R., Pomata, R., & Pisano, M. B. (2012). Antilisterial activity of nisin-like bacteriocin-producing Lactococcus lactis subsp. lactis isolated from traditional Sardinian dairy products. J Biomed Biotechnol, 2012, 376428.
Cossi, M. V. C., Burin, R. C. K., Camargo, A. C., Dias, M. R., Lanna, F. G. P. A., Pinto, P. S. A., & Nero, L. A. (2014). Low occurrence of Salmonella in the beef processing chain from Minas Gerais state, Brazil: From bovine hides to end cuts. Food Control, 40, 320–323.
Dal Bello, B., Cocolin, L., Zeppa, G., Field, D., Cotter, P. D., & Hill, C. (2012). Technological characterization of bacteriocin producing Lactococcus lactis strains employed to control Listeria monocytogenes in cottage cheese. Int J Food Microbiol, 153, 58–65.
Dal Bello, B., Rantsiou, K., Bellio, A., Zeppa, G., Ambrosoli, R., Civera, T., & Cocolin, L. (2010). Microbial ecology of artisanal products from North West of Italy and antimicrobial activity of the autochthonous populations. LWT Food Sci Technol, 43, 1151–1159.
de Arauz, L. J., Jozala, A. F., Mazzola, P. G., & Penna, T. C. V. (2009). Nisin biotechnological production and application: a review. Trends Food Sci Tech, 20, 146–154.
de Kwaadsteniet, M., Todorov, S. D., Knoetze, H., & Dicks, L. M. (2005). Characterization of a 3944 Da bacteriocin, produced by Enterococcus mundtii ST15, with activity against Gram-positive and Gram-negative bacteria. Int J Food Microbiol, 105, 433–444.
de Vuyst, L., Callewaert, R., & Crabbé, K. (1996). Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology, 142, 817–827.
Deegan, L. H., Cotter, P. D., Hill, C., & Ross, P. (2006). Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J, 16, 1058–1071.
Delgado, S., & Mayo, B. (2004). Phenotypic and genetic diversity of Lactococcus lactis and Enterococcus spp. strains isolated from Northern Spain starter-free farmhouse cheeses. Int. J. Food Microbiol, 90, 309–319.
Du Toit, M., Franz, C. M. A. P., Dicks, L. M. T., & Holzapfel, W. H. (2000). Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. J Appl Microbiol, 88, 482–494.
Favaro, L., Basaglia, M., Casella, S., Hue, I., Dousset, X., Franco, B. D. G. M., & Todorov, S. D. (2014). Bacteriocinogenic potential and safety evaluation of non-starter Enterococcus faecium strains isolated from home made white brine cheese. Food Microbiol, 38, 228–239.
Galvez, A., Abriouel, H., Lopez, R. L., & Ben Omar, N. (2007). Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol, 120, 51–70.
Gelsomino, R., Vancanneyt, M., Cogan, T. M., Condon, S., & Swings, J. (2002). Source of enterococci in a farmhouse raw-milk cheese. Appl Environ Microbiol, 68, 3560–3565.
Graves, L. M., & Swaminathan, B. (2001). PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int J Food Microbiol, 65, 55–62.
Gutiérrez-Méndez, N., Rodríguez-Figueroa, J. C., González-Córdova, A. F., Nevárez-Moorillón, G. V., Rivera-Chavira, B., & Vallejo-Cordoba, B. (2010). Phenotypic and genotypic characteristics of Lactococcus lactis strains isolated from different ecosystems. Can J Microbiol, 56, 432–439.
Hadji-Sfaxi, I., El-Ghaish, S., Ahmadova, A., Batdorj, B., le Blay-Laliberté, G., Barbier, G., Haertlé, T., & Chobert, J. M. (2011). Antimicrobial activity and safety of use of Enterococcus faecium PC4.1 isolated from Mongol yogurt. Food Control, 22, 2020–2027.
Han, E. J., Lee, N. K., Choi, S. Y., & Paik, H. D. (2013). Short communication: Bacteriocin KC24 produced by Lactococcus lactis KC24 from kimchi and its antilisterial effect in UHT milk. J Dairy Sci, 96, 101–104.
Hennekinne, J. A., De Buyser, M. L., & Dragacci, S. (2012). Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol Rev, 36, 815–836.
Jurkovic, D., Krizkova, L., Sojka, M., Takacova, M., Dusinsky, R., Krajcovic, J., Vandamme, P., & Vancanneyt, M. (2007). Genetic diversity of Enterococcus faecium isolated from Bryndza cheese. Int J Food Microbiol, 116, 82–87.
Leroy, F., & de Vuyst, L. (2004). Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Tech, 15, 67–78.
Lewus, C. B., & Montville, T. J. (1991). Detection of bacteriocins produced by lactic acid bacteria. J Microbiol Meth, 13, 145–150.
Mannu, L., Paba, A., Pes, M., Floris, R., Scintu, M. F., & Morelli, L. (1999). Strain typing among enterococci isolated from home-made Pecorino Sardo cheese. FEMS Microbiol Lett, 170, 25–30.
Mayr-Harting, A., Hedges, A. J., Berkeley, R. C. W. (1972), in Methods in Microbiology, vol. Volume 7, Part A, (Norris, J. R. and Ribbons, D. W., eds.), Academic Press, pp. 315-422.
Montanhini, M. T. M., Colombo, M., Nero, L. A., & Bersot, L. S. (2013). Short communication: presence of neutral metallopeptidase (npr) gene and proteolytic activity of Bacillus cereus isolated from dairy products. J Dairy Sci, 96, 5641–5643.
Nikolic, M., Terzic-Vidojevic, A., Jovcic, B., Begovic, J., Golic, N., & Topisirovic, L. (2008). Characterization of lactic acid bacteria isolated from Bukuljac, a homemade goat’s milk cheese. Int J Food Microbiol, 122, 162–170.
Nishie, M., Nagao, J.-I., & Sonomoto, K. (2012). Antibacterial peptides “bacteriocins”: an overview of their diverse characteristics and applications. Biocontrol Sci, 17, 1–16.
Okuklu, B. (2005) Investigation of chromosomal and plasmid dna profiles of Lactococcus lactis ssp. lactis. Master, Izmir Institute of Technology, Izmir.
Olasupo, N. A., Schillinger, U., Narbad, A., Dodd, H., & Holzapfel, W. H. (1999). Occurrence of nisin Z production in Lactococcus lactis BFE 1500 isolated from wara, a traditional Nigerian cheese product. Int J Food Microbiol, 53, 141–152.
Ortolani, M. B. T., Yamazi, A. K., Moraes, P. M., Vicosa, G. N., & Nero, L. A. (2010). Microbiological quality and safety of saw milk and soft cheese and detection of autochthonous lactic acid bacteria with antagonistic activity against Listeria monocytogenes, Salmonella spp., and Staphylococcus aureus. Foodborne Pathog Dis, 7, 175–180.
Parente, E., & Ricciardi, A. (1994). Influence of pH on the production of enterocin 1146 during batch fermentation. Lett Appl Microbiol, 19, 12–15.
Parente, E., & Ricciardi, A. (1999). Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl Microbiol Biotechnol, 52, 628–638.
Park, Y. W. (2007). Rheological characteristics of goat and sheep milk. Small Ruminant Res, 68, 73–87.
Passerini, D., Beltramo, C., Coddeville, M., Quentin, Y., Ritzenthaler, P., Daveran-Mingot, M. L., & Le Bourgeois, P. (2010). Genes but not genomes reveal bacterial domestication of Lactococcus Lactis. PLoS ONE, 5, 1–12.
Perin, L. M., Miranda, R. O., Camargo, A. C., Colombo, M., Carvalho, A. F., & Nero, L. A. (2013). Antimicrobial activity of the Nisin Z producer Lactococcus lactis subsp. lactis Lc08 against Listeria monocytogenes in skim milk. Arq Bras Med Vet Zootec, 65, 1554–1560.
Perin, L. M., Moraes, P. M., Viçosa, G. N., Silva, A. J., & Nero, L. A. (2012). Identification of bacteriocinogenic Lactococcus isolates from raw milk and cheese capable of producing nisin A and nisin Z. Int Dairy J, 25, 46–51.
Perin, L. M. and Nero, L. A. (2014) Antagonistic lactic acid bacteria isolated from goat milk and identification of a novel nisin variant Lactococcus lactis. BMC Microbiol. 14.
Psoni, L., Kotzamanidis, C., Yiangou, M., Tzanetakis, N., & Litopoulou-Tzanetaki, E. (2007). Genotypic and phenotypic diversity of Lactococcus lactis isolates from Batzos, a Greek PDO raw goat milk cheese. Int J Food Microbiol, 114, 211–220.
Randazzo, C. L., Caggia, C., & Neviani, E. (2009). Application of molecular approaches to study lactic acid bacteria in artisanal cheeses. J Microbiol Meth, 78, 1–9.
Rehaiem, A., Martinez, B., Manai, M., & Rodriguez, A. (2010). Production of enterocin A by Enterococcus faecium MMRA isolated from 'Rayeb', a traditional Tunisian dairy beverage. J Appl Microbiol, 108, 1685–1693.
Rodriguez, E., Gonzalez, B., Gaya, P., Nunez, M., & Medina, M. (2000). Diversity of bacteriocins produced by lactic acid bacteria isolated from raw milk. Int Dairy J, 10, 7–15.
Schirru, S., Todorov, S. D., Favaro, L., Mangia, N. P., Basaglia, M., Casella, S., Comunian, R., Franco, B. D. G. D., & Deiana, P. (2012). Sardinian goat’s milk as source of bacteriocinogenic potential protective cultures. Food Control, 25, 309–320.
Settanni, L., Guarcello, R., Gaglio, R., Francesca, N., Aleo, A., Felis, G. E., & Moschetti, G. (2014). Production, stability, gene sequencing and in situ anti-Listeria activity of mundticin KS expressed by three Enterococcus mundtii strains. Food Control, 35, 311–322.
Stenfors, L. P. A., Fagerlund, A., & Granum, P. E. (2008). From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev, 32, 579–606.
Stern, N. J., Svetoch, E. A., Eruslanov, B. V., Perelygin, V. V., Mitsevich, E. V., Mitsevich, I. P., Pokhilenko, V. D., Levchuk, V. P., Svetoch, O. E., & Seal, B. S. (2006). Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Ch, 50, 3111–3116.
Strompfová, V., & Lauková, A. (2007). In vitro study on bacteriocin production of Enterococci associated with chickens. Anaerobe, 13, 228–237.
Todorov, S. D. (2008). Bacteriocin production by Lactobacillus plantarum AMA-K isolated from Amasi, a Zimbabwean fermented milk product and study of the adsorption of bacteriocin AMA-K to Listeria sp. Braz J Microbiol, 39, 178–187.
Todorov, S. D., & Dicks, L. M. T. (2009). Bacteriocin production by Pediococcus pentosaceus isolated from marula (Scerocarya birrea). Int J Food Microbiol, 132, 117–126.
Todorov, S. D., & Dicks, L. M. T. (2009). Effect of modified MRS medium on production and purification of antimicrobial peptide ST4SA produced by Enterococcus mundtii. Anaerobe, 15, 65–73.
Todorov, S. D., Wachsman, M., Tome, E., Dousset, X., Destro, M. T., Dicks, L. M., Franco, B. D., Vaz-Velho, M., & Drider, D. (2010). Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol, 27, 869–879.
Turabelidze, D., Kotetishvili, M., Kreger, A., Morris, J. G., & Sulakvelidze, A. (2000). Improved pulsed-field gel electrophoresis for typing vancomycin-resistant enterococci. J Clin Microbiol, 38, 4242–4245.
Viçosa, G. N., Moraes, P. M., Yamazi, A. K., & Nero, L. A. (2010). Enumeration of coagulase and thermonuclease-positive Staphylococcus spp. in raw milk and fresh soft cheese: An evaluation of Baird-Parker agar, Rabbit Plasma Fibrinogen agar and the Petrifilm™ Staph Express count system. Food Microbiol, 27, 447–452.
Acknowledgments
The authors are thankful to CNPq, CAPES, FAPESP, and FAPEMIG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cavicchioli, V.Q., Dornellas, W.d.S., Perin, L.M. et al. Genetic Diversity and Some Aspects of Antimicrobial Activity of Lactic Acid Bacteria Isolated from Goat Milk. Appl Biochem Biotechnol 175, 2806–2822 (2015). https://doi.org/10.1007/s12010-015-1511-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1511-8