Abstract
Background
Earlier results with porous tantalum acetabular cups in revision THA generally have been favorable. Recently there has been some evidence presented that porous tantalum cups might decrease the risk of rerevision in the setting of revision hip surgery performed owing to prosthetic joint infection (PJI). As the data supporting this assertion come from a study with a limited study population, examining this issue with a large registry approach may be enlightening.
Questions/purposes
By combining results from two large, national registries, we asked: (1) Do porous tantalum cups show improved survival after revision THA compared with other cementless designs? (2) Does the use of porous tantalum cups influence survivorship when rerevision for PJI is the endpoint?
Methods
A total of 2442 first-time THA revisions with porous tantalum cups and 4401 first-time revisions with other uncemented cups were included in this collaborative study between the Australian and Swedish national joint registries. The mean age of the patients was 69 years (range, 19–97 years), 3754 (55%) of the patients were women, and the mean followup for the porous tantalum and uncemented control groups were 3.0 years (SD, ± 2.1 years) and 3.4 years (SD, ± 2.3 years), respectively. Concomitant stem revision was more common in the porous tantalum group (43% versus 36%). The use of porous tantalum augments also was analyzed as a proxy for more complex acetabular reconstructions. In an attempt to further reduce selection bias, we performed subgroup analysis for primary operations attributable to osteoarthritis and first revision attributable to aseptic loosening.
Results
Kaplan-Meier survivorship with rerevisison for any reason up to 7 years was comparable between the porous tantalum cup group and the uncemented cup control group (86% [95% CI, 85%–89%] and 87% [95% CI, 85%–89%], respectively; p = 0.85) and the overall survivorship up to 7 years with a second revision for PJI as the endpoint (97% [95% CI, 95%–98%] and 97% [95% CI, 96%–98%], respectively; p = 0.64). Excluding procedures where augments had been used or studying primary osteoarthritis and first revision owing to aseptic loosening subgroups did not change this result.
Conclusions
Implant survival for a porous tantalum cup in first-time THA revision was similar to the survival of the uncemented cup control group. With the numbers available, no benefit in survival with rerevision for infection as the endpoint could be ascribed to the porous tantalum cup group, as has been suggested by earlier work. Further studies with acetabular bone deficiency data, greater insight into host comorbidity factors, and a longer followup are needed to corroborate or refute these results.
Level of Evidence
Level III, therapeutic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research suggesting inferior implant survival when cemented acetabular components are used in revision THA led to increased use of uncemented acetabular cups in first-time revision THA during the last two decades [17]. Although uncemented cups made from porous tantalum initially were designed to help manage challenging bone deficiencies, there is evidence that they are seeing wide use for revision and primary THAs [11, 13]. Porous tantalum has been shown to provide higher porosity, increased initial stability, and better bone ingrowth qualities [2]. Several studies have presented similar or improved results for porous tantalum cups in acetabular revision compared with other uncemented revision cups [5, 8, 12], and the use of porous tantalum cups has been steadily increasing [21].
Risk for prosthetic joint infection (PJI) is higher after revision THA than after primary THA [7, 16]. PJI is the one of the most-common reasons for second or subsequent revision procedures after revision THA, and the economic burden caused by the treatment of PJI is enormous [6, 10, 21]. In a recent single-center study, the use of porous tantalum was associated with a decreased infection rate after revision THA [22]. A possible reduction in infection rate after revision THA would be a great benefit from the economic and patient perspectives. However, data supporting the assumption that porous tantalum as a material could be protective of PJI come from a limited study population and examining this from a large registry approach may be enlightening.
To understand these questions in a large, generalizable population, we used the national total joint registries of Australia and Sweden in a collaborative registry study to ask: (1) Do porous tantalum cups show improved survival after revision THA compared with other cementless designs? (2) Does the use of porous tantalum cups influence survivorship when a rerevision for PJI is the endpoint?
Materials and Methods
Data for this collaborative study were collected from two national, high-quality registers: the Swedish Hip Arthroplasty Register (SHAR) and the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR).
SHAR has been collecting data on THAs since 1979 and currently has data on approximately 40,000 revisions. In Sweden all orthopaedic units performing revision hip arthroplasties report to SHAR. The completeness of the reoperations has been reported at 90% [14]. Descriptive surgical data are initially completed on standard forms by the responsible surgical team at each center. The operation notes subsequently are sent to SHAR. Register coordinators at SHAR perform quality control and complete the coding based on the operation notes provided.
The AOANJRR began data collection in 1999 and reached full national coverage in 2002. AOANJRR data are validated against patient-level data provided by each state and territory health department in Australia using a sequential, multilevel matching process. Data also are matched biannually with the National Death Index to obtain information regarding the date of death [1].
As a result of these validation processes and quality controls, data from SHAR and AOANJRR are reliable for primary and revision arthroplasties, including revisions resulting from infection.
Data included in this study start from January 1, 2006, which is the time when reporting of porous tantalum cups started in both registries. To determine if there were any substantial differences in implant survival for the porous tantalum or other uncemented cups being reported to the two registries, we performed regression analysis with data stratified for the SHAR and AOANJRR. The stratified analysis did not alter the results of the Cox regression analysis on either of our study’s two main research questions.
Between January 2006 and December 2014, 12,272 and 11,333 first-time THA revisions were reported to SHAR and AOANJRR, respectively. During the aforementioned period, in total 2442 acetabular revisions performed with a porous tantalum design were registered in SHAR (1456) and AOANJRR (981). The five most-frequently used uncemented cup designs, six different cup designs in total, from each registry (uncemented, n = 4401; 1793 from SHAR and 2608 from the AAONJRR) were identified as the uncemented control group (Table 1). Cup-only and total (femoral component and cup) first-time revisions, in which porous tantalum or the other five most-frequently used uncemented cups from each registry were used, were included. Patient selection is shown in more detail in a flowchart (Fig. 1).
The mean age of the patients at the time of the index revision was 69 years (range, 19–97 years) and 55% (3754) of them were women in both groups (Table 2). Aseptic loosening was the most common reason for first-time revision in the porous tantalum group (1679 operations; 69% of all first-time revisions with a porous tantalum cup) and in the uncemented control group (2279 operations; 52% of all first-time revisions in the uncemented control group) (Table 2). Infection accounted for 3% (85 operations) of first-time revisions in the porous tantalum group and 4% (187 operations) in the uncemented control group.
Concomitant stem revision was more common in the porous tantalum group (1047 operations; 43%) compared with the uncemented control group (1592 operations; 36%). The mean followup was 3 years (range, 0–9 years) in the porous tantalum group and 3.4 years (range, 0–9 years) in the uncemented control group, with numbers at risk decreasing during the 8-year followup (Table 3).
Statistical Analysis
The time to second revision was defined using Kaplan-Meier estimates of survivorship. Survival curves were truncated when numbers at risk in any of the groups fell below 100 cases. All analyses were performed using R statistical software (RStudio: Integrated Development for R; RStudio, Inc, Boston, MA, USA). Log-rank test was used to compare the survival up to 7 years. Two separate Cox regression models, adjusted for age and sex, were used. In the first model, all revisions were included and in the second model, only patients with primary arthritis who underwent surgery owing to aseptic loosening were included (n = 3211). The primary outcome was second revision for any reason and the secondary outcome was second revision resulting from infection. Survival data are presented as percentage with SD. Cox regression analysis is presented with hazard ratio (HR) and 95% CI. Probability values less than 0.05 were set to define statistical significance.
Ethical Approval
This study was approved by Regionala Etikprovningsnamnden i Goteborg (study number 669-16).
Results
Overall Survivorship
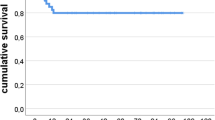
Survival of the cup with rerevision as a result of all causes up to 7 years was comparable between the porous tantalum group (86%; 95% CI, 83%–89%) and the uncemented control group (87%; 95% CI, 86%–89%; p = 0.85) (Fig. 2).
Survivorship Free From Rerevision for Infection
Using rerevision attributable to infection as the endpoint, the survival of the porous tantalum group (97%; 95 CI, 96%–99%) was comparable to that of the uncemented cup control group (97%; 95% CI, 96%–98%; p = 0.64) (Fig. 3). Only 272 hips underwent a first revision owing to infection, and of these, 13 had rerevision resulting from infection. Because of the small subgroup size, we were not able to perform a statistically meaningful analysis for this subgroup.
After adjusting data for age and sex in the Cox model, the risk of rerevision was comparable between the two groups using either all rerevisions or infection necessitating rerevision as the endpoint (Table 4). Analyzing a subgroup of patients with primary osteoarthritis as the indication for primary THA who underwent revision surgery owing to aseptic loosening of the cup, the HRs for rerevision resulting from all causes and rerevision resulting from infection did not differ between porous tantalum and other uncemented cups (Table 4). In addition, to further reduce selection bias, we performed a separate analysis excluding cases in which acetabular augments were used (n = 209) and controlled for hospital-level data. Since augments typically are used for large bone defects which might compromise the outcome, by excluding cases where augments had been used we aimed to exclude patients with the largest bone defects and thereby reduce selection bias between the porous tantalum and other uncemented cups groups. This did not have any effect on the results (Table 5). We also stratified the Cox regression analysis for hospitals using porous tantalum cups in more or less than 50% of the cases and this stratification also did not alter the risk of rerevision resulting from all causes (HR, 1.1; 95% CI, 0.9–1.3; p = 0.2) or infection (HR, 1.1; 95% CI, 0.8–1.5; p = 0.6).
Discussion
The incidence of revision THA continues to increase. According to some studies, the incidence of revision THA might rise by 300% from 2012 to 2030 [9, 15]. Good short-term results have supported the use of porous tantalum cups in revision THA [4, 18, 19]. In the current collaborative registry study, we compared first revision results between the porous tantalum cup and the other most commonly used uncemented titanium revision cups. In our multinational registry-based databases, porous tantalum cups had a comparable rerevision rate with the five most frequently used uncemented revision cups when revision for any reason was used as the endpoint. The use of a porous tantalum cup for revision also did not decrease risk of a rerevision resulting from infection, compared with the uncemented cup control group.
We acknowledge that our study has some limitations. First, our analysis is based on registry data and we were unable to determine severity of acetabular bone deficiencies before first revision. It is possible that porous tantalum cups were used in settings of greater bone deficiency, potentially putting them at higher risk of subsequent rerevision. To reduce this bias, we performed a separate subgroup analysis excluding more severe cases (based on use of augments) and controlling for hospital level data. In addition, it is impossible to assess radiographs for a large registry-based population. Radiographic analysis might have provided more information regarding the current status of nonrevised acetabular components. Second, our data did not include any patient-reported outcome measures and it is possible that some of the hips might be symptomatic, although they have not been revised. Third, our mean followup was only 3 years, which is a relatively short time in a hip implant’s lifespan. However, uncemented revision cups more often are associated with higher early revision rates, whereas cemented cups often are associated with later wear-related problems [11]. In addition, the risk of rerevision resulting from infection is higher during the early period after the first revision [14]. Because of these reasons, we believe it is important to report our early results with these implants. Further, our control group of other uncemented cups was heterogeneous. However, our aim was to study whether porous tantalum as a material would have superior outcome compared with other uncemented cups. As there was no superiority in the survival of the porous tantalum group we do not believe that potential individual cup differences in survival in the control group are of any significance. Finally, we were not able to perform a more-detailed analysis on rerevision for infection after first revision for infection because of the small numbers. Future collaborative studies would require greater numbers to gain more insight in this area.
In our study, risk of rerevision for any cause or for infection did not differ between the porous tantalum group and the uncemented control group. Results have been reported in which rerevision rates after acetabular revision with the porous tantalum cup have been lower compared with the results of other uncemented revision cup designs [5, 22]. In other studies, however, there has not been a difference in the short- and mid-term survivorship between porous tantalum and other uncemented cups [8, 12]. Our data are based on registry data and we were unable to determine the severity of acetabular defects before primary revision. There is evidence that porous tantalum cups have good outcome results when treating larger acetabular defects [20, 23], and it is possible that porous tantalum cups may have been used for more-severe bone deficiencies as a result of the perceived biologic advantages of the material in this setting. This selection bias might have skewed the results. Both registries have acetabular augment use information available, and to evaluate this possible bias in case mix, we performed a subgroup analysis for data including and excluding patients with augments. This did not change the results. Unfortunately, we were unable to obtain data documenting bone graft use. Furthermore, patients with larger bone deficiencies might be overall more fragile and have more comorbidities than patients with better bone health. Being able to assess comorbidity data also might have reduced this possible bias.
In our analysis, including 6843 first-time revisions, the infection rate after first revision did not differ between porous tantalum and titanium revision cups. Infection after revision THA is a major problem and PJI prevalence is significantly higher after revision THA than after primary THA [7, 16, 24]. Cost for revision surgery has been reported to be 30% to 100% higher than for primary THA and revision for PJI is thus a major economic burden for society [3, 6, 10]. In addition, PJI has a major effect on a patient’s quality of life. In a recent study by Tokarski et al. [22], use of porous tantalum cups instead of titanium cups was associated with a lower overall rerevision rate and a lower rerevision incidence resulting from infection after first revision. Revision rates were 5% and 10% for rerevision for any reason and 3% and 18% for rerevisions resulting from infection, respectively [22]. They discussed that the decreased infection rate observed might be the result of better osseointegration or that tantalum might have some antibacterial qualities [22]. In their study, patients were followed for a minimum of 90 days. In cases revised owing to aseptic loosening (n = 846), there were no differences between the tantalum and the titanium design when septic failure was used as the endpoint (3%). However, when index revision was performed owing to infection (n = 144), the risk of septic failure was 18% (n = 14) for the titanium cups and 3% (n = 2) for the tantalum cups. We were unable to replicate the analysis Tokarski et al. used for rerevision for infection in this much-larger registry study. In our study, the number of index revisions resulting from infection (n = 272) and rerevisions resulting from infection in this group (n = 13) was too low to allow meaningful statistical analysis of this subcohort.
Implant survival for porous tantalum cups was similar to that of an uncemented control group for first-time revision. We were unable to identify a “protective effect” of porous tantalum cups regarding rerevision for infection. However, additional followup with information regarding severity of bone deficiency is needed to limit selection bias and to determine mid- to long-term results for the porous tantalum cups.
References
Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2015 - Hip and Knee Arthroplasty. Available at: https://aoanjrr.sahmri.com/annual-reports-2016. Accessed May 10, 2017.
Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81:907–914.
Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620.
Davies JH, Laflamme GY, Delisle J, Fernandes J. Trabecular metal used for major bone loss in acetabular hip revision. J Arthroplasty. 2011;26:1245–1250.
Jafari SM, Bender B, Coyle C, Parvizi J, Sharkey PF, Hozack WJ. Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin Orthop Relat Res. 2010;468:459–465.
Kamath AF, Ong KL, Lau E, Chan V, Vail TP, Rubash HE, Berry DJ, Bozic KJ. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30:1492–1497.
Kosashvili Y, Backstein D, Safir O, Lakstein D, Gross AE. Dislocation and infection after revision total hip arthroplasty: comparison between the first and multiply revised total hip arthroplasty. J Arthroplasty. 2011;26:1170–1175.
Kremers HM, Howard JL, Loechler Y, Schleck CD, Harmsen WS, Berry DJ, Cabanela ME, Hanssen AD, Pagnano MW, Trousdale RT, Lewallen DG. Comparative long-term survivorship of uncemented acetabular components in revision total hip arthroplasty. J Bone Joint Surg Am. 2012;94:e82.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785.
Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61–65.e1.
Mohaddes M, Garellick G, Kärrholm J. Method of fixation does not influence the overall risk of rerevision in first-time cup revisions. Clin Orthop Relat Res. 2013;471:3922–3931.
Mohaddes M, Rolfson O, Kärrholm J. Short-term survival of the trabecular metal cup is similar to that of standard cups used in acetabular revision surgery. Acta Orthop. 2015;86:26–31.
Noiseux NO, Long WJ, Mabry TM, Hanssen AD, Lewallen DG. Uncemented porous tantalum acetabular components: early follow-up and failures in 613 primary total hip arthroplasties. J Arthroplasty. 2014;29:617–620.
Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24(6 suppl):105–109.
Patel A, Pavlou G, Mújica-Mota RE, Toms AD. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Joint J. 2015;97:1076–1081.
Phillips CB, Barrett JA, Losina E, Mahomed NN, Lingard EA, Guadagnoli E, Baron JA, Harris WH, Poss R, Katz JN. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85:20–26.
Pulido L, Rachala SR, Cabanela ME. Cementless acetabular revision: past, present, and future. Revision total hip arthroplasty: the acetabular side using cementless implants. Int Orthop. 2011;35:289–298.
Siegmeth A, Duncan CP, Masri BA, Kim WY, Garbuz DS. Modular tantalum augments for acetabular defects in revision hip arthroplasty. Clin Orthop Relat Res. 2009;467:199–205.
Skyttä ET, Eskelinen A, Paavolainen PO, Remes VM. Early results of 827 trabecular metal revision shells in acetabular revision. J Arthroplasty. 2011;26:342–345.
Sternheim A, Backstein D, Kuzyk PR, Goshua G, Berkovich Y, Safir O, Gross AE. Porous metal revision shells for management of contained acetabular bone defects at a mean follow-up of six years: a comparison between up to 50% bleeding host bone contact and more than 50% contact. J Bone Joint Surg Br. 2012;94:158–162.
Svenska Höftprotesregistret [Swedish Hip Arthroplasty Register]. Annual Report 2013. Available at: https://shpr.registercentrum.se/publikationer/publikationer/p/Hys5hJaLg. Accessed May 10, 2017.
Tokarski AT, Novack TA, Parvizi J. Is tantalum protective against infection in revision total hip arthroplasty? Bone Joint J. 2015;97:45–49.
Weeden SH, Schmidt RH. The use of tantalum porous metal implants for Paprosky 3A and 3B defects. J Arthroplasty. 2007;22(6 suppl 2):151–155.
Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89:526–533.
Author information
Authors and Affiliations
Corresponding author
Additional information
One of the authors certifies that he (MM), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from Zimmer Biomet (Warsaw, IN, USA), not related to this study.
One of the authors certifies that he (HM), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of amount of USD 10,000–100,000 from MAKO Surgical Corporation (Ft Lauderdale, FL, USA) and an amount of USD 10,000–100,000 from Stryker Corporation (Kalamazoo, MI, USA), not related to this study.
One of the authors certifies that he (OR), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000–100,000 from the International Consortium for Health Outcomes Measurement (Cambridge, MA, USA)) not related to this study.
The institution of one or more of the authors (HM, MM, OR) has received funding from Zimmer Biomet, DePuy, MAKO, and LINK Sweden not related to this study.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
The Regionala Etikprovningsnamnden i Goteborg approved the human protocol for this investigation, and each author certifies that all investigations were conducted in conformity with ethical principles of research.
This work was performed in Sahlgrenska University Hospital, Gothenburg, Sweden; Swedish Hip Arthroplasty Register, Gothenburg, Sweden; Australian Orthopaedic Association National Joint Replacement Registry, Adelaide, Australia; Harris Orthopaedic Laboratory, Boston, MA, USA; Copenhagen University Hospital Hvidore, Copenhagen, Denmark; Danish Hip Arthroplasty Register, Aarhus, Denmark; Finnish Arthroplasty Register, Helsinki, Finland; and Turku University Hospital, Turku, Finland.
About this article
Cite this article
Laaksonen, I., Lorimer, M., Gromov, K. et al. Does the Risk of Rerevision Vary Between Porous Tantalum Cups and Other Cementless Designs After Revision Hip Arthroplasty?. Clin Orthop Relat Res 475, 3015–3022 (2017). https://doi.org/10.1007/s11999-017-5417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-017-5417-3