Abstract
Background
Extremity trauma is the most common injury seen in combat hospitals as well as in civilian trauma centers. Major skeletal muscle injuries that are complicated by ischemia often result in substantial muscle loss, residual disability, or even amputation, yet few treatment options are available. A therapy that would increase skeletal muscle tolerance to hypoxic damage could reduce acute myocyte loss and enhance preservation of muscle mass in these situations.
Questions/purposes
In these experiments, we investigated (1) whether cobalt protoporphyrin (CoPP), a pharmacologic inducer of cytoprotective heme oxygenase-1 (HO-1), would upregulate HO-1 expression and activity in skeletal muscle, tested in muscle-derived stem cells (MDSCs); and (2) whether CoPP exposure would protect MDSCs from cell death during in vitro hypoxia/reoxygenation. Then, using an in vivo mouse model of hindlimb ischemia/reperfusion injury, we examined (3) whether CoPP pharmacotherapy would reduce skeletal muscle damage when delivered after injury; and (4) whether it would alter the host inflammatory response to injury.
Methods
MDSCs were exposed in vitro to a single dose of 25 μΜ CoPP and harvested over 24 to 96 hours, assessing HO-1 protein expression by Western blot densitometry and HO-1 enzyme activity by cGMP levels. To generate hypoxia/reoxygenation stress, MDSCs were treated in vitro with phosphate-buffered saline (vehicle), CoPP, or CoPP plus an HO-1 inhibitor, tin protoporphyrin (SnPP), and then subjected to 5 hours of hypoxia (< 0.5% O2) followed by 24 hours of reoxygenation and evaluated for apoptosis. In vivo, hindlimb ischemia/reperfusion injury was produced in mice by unilateral 2-hour tourniquet application followed by 24 hours of reperfusion. In three postinjury treatment groups (n = 7 mice/group), CoPP was administered intraperitoneally during ischemia, at the onset of reperfusion, or 1 hour later. Two control groups of mice with the same injury received phosphate-buffered saline (vehicle) or the HO-1 inhibitor, SnPP. Myocyte damage in the gastrocnemius and tibialis anterior muscles was determined by uptake of intraperitoneally delivered Evans blue dye (EBD), quantified by image analysis. On serial sections, inflammation was gauged by the mean myeloperoxidase staining intensity per unit area over the entirety of each muscle.
Results
In MDSCs, a single exposure to CoPP increased HO-1 protein expression and enzyme activity, both of which were sustained for 96 hours. CoPP treatment of MDSCs reduced apoptotic cell populations by 55% after in vitro hypoxia/reoxygenation injury (from a mean of 57.3% apoptotic cells in vehicle-treated controls to 25.7% in CoPP-treated cells, mean difference 31.6%; confidence interval [CI], 28.1–35.0; p < 0.001). In the hindlimb ischemia/reperfusion model, CoPP delivered during ischemia produced a 38% reduction in myocyte damage in the gastrocnemius muscle (from 86.4% ± 7% EBD+ myofibers in vehicle-treated, injured controls to 53.2% EBD+ in CoPP-treated muscle, mean difference 33.2%; 95% CI, 18.3, 48.4; p < 0.001). A 30% reduction in injury to the gastrocnemius was seen with drug delivery at the onset of reperfusion (to 60.6% ± 13% EBD+ with CoPP treatment, mean difference 25.8%; CI, 12.2–39.4; p < 0.001). In the tibialis anterior, however, myocyte damage was decreased only when CoPP was given at the onset of reperfusion, resulting in a 27% reduction in injury (from 78.8% ± 8% EBD+ myofibers in injured controls to 58.3% ± 14% with CoPP treatment, mean difference 20.5%; CI, 6.1–35.0; p = 0.004). Delaying CoPP delivery until 1 hour after tourniquet release obviated the protective effect in both muscles. Mean MPO staining intensity per unit area, indicating the host inflammatory response, decreased by 27–34% across both the gastrocnemius and tibialis anterior muscles when CoPP was given either during ischemia or at the time of reperfusion. Delaying drug delivery until 1 hour after the start of reperfusion abrogated this antiinflammatory effect.
Conclusions
CoPP can decrease skeletal muscle damage when given early in the course of ischemia/reperfusion injury and also provide protection for regenerative stem cell populations.
Clinical Relevance
Pharmacotherapy with HO-1 inducers, delivered in the field, on hospital arrival, or during trauma surgery, may improve preservation of muscle mass and muscle-inherent stem cells after severe ischemic limb injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extremity trauma accounts for over 70% of battlefield injuries [36] and is also the most common injury seen in civilian trauma centers. Morbidity is high when limb injury is complicated by ischemia, often resulting in residual disability or even amputation [26, 32]. Skeletal muscle has a lower threshold for ischemic damage than nerve, skin, or bone [3]; thus, even transient ischemia can cause substantial impairment. Furthermore, because most myocyte death occurs during reperfusion [34], ongoing muscle loss may continue after restoration of blood flow and resolution of ischemia. A means to improve the survival of skeletal myocytes in the face of ischemia/reperfusion injury would enhance the preservation of muscle mass and, in turn, the prospects for functional recovery.
In ischemic heart disease, it is well established that preconditioning the heart can protect cardiomyocytes from cell death during subsequent myocardial ischemia [23]. Preconditioning is categorized as either “early” or “late” based on the timing and duration of the effect. Although early preconditioning provides protection from ischemic events occurring within minutes to hours, late preconditioning, operating through different mediators, can impart cell protective effects lasting 1 to 4 days [37]. If similarly operative in skeletal muscle, this 1- to 4-day timeframe would be sufficient to preserve ischemic skeletal muscle during the most acute postinjury period.
One recognized endogenous mediator of late preconditioning in cardiac muscle is heme oxygenase-1 (HO-1) [24]. In the heart, HO-1 overexpression by preemptive gene therapy reduces myocardial infarct size [21]. Furthermore, pretreatment with cobalt protoporphyrin (CoPP), a pharmacologic inducer of HO-1, improves the survival of cardiomyocyte grafts implanted into ischemic myocardial infarcts [13, 18] and enhances donor organ viability in experimental heart transplantation [12].
Some existing evidence supports the premise that HO-1 induction might be adapted for use in skeletal muscle. A previous study in rats showed that preemptive skeletal muscle gene transfer using HO-1-expressing viral vectors reduced skeletal muscle loss from ischemic injury sustained 5 weeks later [25]. Also, in both rodents [20, 31] and humans [28], HO-1 has been identified as one of only a few genes upregulated by physical exercise that provides protection against later injury-induced muscle damage.
The purpose of the following experiments was to test whether pharmacologic induction of HO-1 expression could be used therapeutically in skeletal muscle to increase myocyte salvage after severe ischemia/reperfusion injury. We first asked (1) whether CoPP would upregulate HO-1 expression and activity in skeletal muscle, tested in muscle-derived stem cells (MDSCs); and (2) whether CoPP exposure would, in turn, protect MDSCs from cell death during in vitro hypoxia/reoxygenation. Then, using an in vivo mouse model, we asked (3) whether CoPP pharmacotherapy would reduce skeletal muscle damage in hindlimb ischemia/reperfusion injury; and (4) whether it would alter the host inflammatory response to injury. Importantly, because pretreatment, or preconditioning, is not an option in trauma care, we examined the critical question of whether drug delivery after injury, or “postconditioning,” would be an effective means of preserving muscle mass.
Materials and Methods
HO-1 Induction in MDSCs
MDSCs were isolated from skeletal muscle of 3-week-old C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) by sequential preplating, replicating previously described techniques [29]. MDSCs were then propagated on Type I collagen (Sigma-Aldrich, St Louis, MO, USA)-coated flasks in proliferation medium (DMEM supplemented with 10% horse serum, 10% fetal bovine serum, 0.5% chicken embryo extract, and 1% penicillin–streptomycin [all from Life Technologies, Grand Island, NY, USA]). To determine whether CoPP would induce HO-1 expression in MDSCs, MDSCs were cultured for 24 hours in proliferation medium supplemented with 25 μM CoPP (Frontier Scientific, Logan, UT, USA) [13] in phosphate-buffered saline (PBS), PBS alone (vehicle-only control), or 25 μM CoPP plus 25 μM tin protoporphyrin (SnPP) (Frontier Scientific) [13], a specific inhibitor of HO-1 enzymatic activity [22]. Cells were then washed with PBS, placed into fresh proliferation medium without CoPP/SnPP supplementation, and harvested every 24 hours for 96 hours, examining four replicates for each condition and time point.
Hypoxia/reoxygenation Protocol in MDSCs
To mimic tissue ischemia and lack of blood flow, MDSCs were subjected to hypoxia plus nutritional deprivation. MDSCs (2 × 105/well) were cultured under normoxic conditions (21% O2) for 72 hours in proliferation media containing 25 μM CoPP, PBS, or 25 μM CoPP plus 25 μM SnPP. Then, cells were washed with PBS and placed under hypoxic conditions (< 0.5% O2, 37° C) for 5 hours in an anaerobic chamber in hypoxic media (no-glucose DMEM [Gibco 11966-025] with antibiotics but without sera/extracts), which had been equilibrated in the chamber. To simulate reperfusion, cells were washed and returned to standard proliferation media (with both glucose and serum restitution) under normoxic conditions for 16 hours of reoxygenation. Cells were collected for analysis immediately before hypoxia, at the end of hypoxia, and at the end of reoxygenation with four replicates per condition. Harvested cells included both the cells in the supernatant and adherent cells detached by a 5-minute exposure to TE buffer (Gibco 11493; Life Technologies). After washing with PBS and centrifugation, cell pellets were fixed in 20% ethanol and stored at −20° C until analysis. Experiments were repeated twice.
HO-1 Expression, Activity Levels, and Apoptosis in MDSCs
Cell pellets were lysed by placing them in 100 μL protein extraction buffer (150 mM EDTA, 1% Nonidet P-40, 20 mM TrisHCl, pH 7.5) plus a protease inhibitor cocktail (P8340; Sigma-Aldrich) for 10 minutes at 4° C. After vortexing, brief sonication, and centrifugation, protein samples were collected from the supernatant. Western blots of total MDSC protein were probed with antibodies to HO-1 (Enzo Life Sciences, Farmingdale, NY, USA) and β-actin. Band intensities for HO-1 relative to β-actin were determined by densitometry using ImageJ (NIH, Bethesda, MD, USA). Levels of cyclic guanosine monophosphate (cGMP) [22], resulting from carbon monoxide, an end product of HO-1 catabolism of heme [2], were measured by enzyme-linked immunosorbent assay (GE Healthcare Life Sciences, Piscataway, NJ, USA) as an index of HO-1 enzyme activity [13].
To quantify apoptosis, ethanol-fixed MDSCs were incubated for 1 hour with 5 μg/mL propidium iodide and then analyzed by flow cytometry [30]. Apoptotic cells were distinguished by hypodiploidy and their prevalence calculated as a percentage of total DNA content.
Hindlimb Ischemia/reperfusion Injury
Animal experiments were approved by the Animal Care and Use Committee of the Benaroya Research Institute and performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). In all experiments, C57BL/6J mice (8–10 weeks of age) were anesthetized with intraperitoneal tribromoethanol (500 mg/kg). To produce acute hindlimb ischemia, a 6-ounce orthodontic rubber band (ORB) was applied as a tourniquet to the left proximal hindlimb using a McGivney hemorrhoidal ligator [8], left in place for 2 hours, and then released for 24 hours of reperfusion.
Initially, a pilot study was conducted to compare the severity of limb ischemia generated by ORB tourniquet application to standard surgical femoral artery ligation. Laser Doppler perfusion imaging revealed that ORB placement completely blocked blood flow to the hindlimb, including collateral flow, with an efficacy equivalent to suture ligation (Supplementary Fig. 1 [Supplemental materials are available with the online version of CORR®.]). Ischemia remained constant over the 2 hours and reversed on release of the ORB or ligature.
For the formal experiments, mice, all with hindlimb ischemia/reperfusion injury, were assigned to five treatment groups (n = 7/group). CoPP (5 mg/kg [15]) was administered intraperitoneally to three therapeutic groups at various time points, designed to model clinically relevant points for medical intervention. In Group 1, CoPP was given during the ischemic period, 30 minutes after tourniquet application; in Group 2, at the onset of reperfusion (on tourniquet removal); and in Group 3, 1 hour after tourniquet removal. In two control groups, intraperitoneal PBS (vehicle only) or SnPP (25 mg/kg [40]) was delivered 24 hours before ischemia/reperfusion injury. For SnPP, this timing was designed to inhibit any baseline or induced HO-1 enzyme activity. The uninjured contralateral limbs in all treatment groups served as additional controls.
After 2 hours of ischemia, buprenorphine (0.05 mg/kg) was administered subcutaneously, the tourniquet removed, Evans blue dye (EBD) (50 μg/g body weight [38]; Sigma-Aldrich) injected intraperitoneally, and mice recovered from anesthesia. Twenty-four hours later, mice were euthanized, and both hindlimbs were excised and fixed for 72 hours in 10% buffered formalin.
Histology and Immunohistochemistry
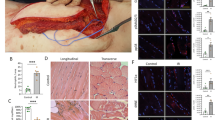
The gastrocnemius and tibialis anterior (TA) muscles were dissected from both hindlimbs and paraffin-embedded. The muscles were cut into longitudinal and transverse sections at their midpoints, then deparaffinized, hydrated, and stained with hematoxylin and eosin. EBD uptake was used to identify myofibers with permeabilized cell membranes [11, 46], indicative of sarcolemmal disruption and myofiber degeneration. Composite images, which consisted of all muscle-containing fields over the full length and width of each muscle, were surveyed for EBD uptake under fluorescence microscopy (540/610 nm) using SPOT software (Spot Imaging Solutions, Sterling Heights, MI, USA). The percentage of the total muscle cross-sectional area consisting of EBD-positive myofibers was quantified by a blinded observer (H-MPW) using ImageJ.
Myeloperoxidase (MPO) immunostaining intensity on adjacent serial sections was used to gauge the effect of CoPP pharmacotherapy on the host inflammatory response. Positive staining consisted not only of intracellular MPO within activated neutrophils [34], but also included MPO released within the extracellular space and permeating some myocytes. To ensure comparability, all tissue sections for MPO staining were processed as a single batch in a Bond Max automated system (Leica Biosystems, Buffalo Grove, IL, USA) using cover tiles to ensure even distribution of antibodies and reagents. Sections were heat-treated for antigen retrieval, then incubated with a rabbit polyclonal antibody to MPO (Abcam, Cambridge, MA, USA) followed by a peroxidase-conjugated secondary antibody (Vector Labs, Burlingame, CA, USA), visualized with 3,3′-diaminobenzidine chromogen (R&D Systems, Minneapolis, MN, USA), and counterstained with hematoxylin. All fields comprising each section were imaged on the same day under identical conditions and settings using SPOT software (Spot Imaging Solutions) and saved in 32-bit TIFF formats. As with the EBD assessments, composite images covering the entirety of each muscle were constructed and converted to grayscale for analysis. Staining intensities were recorded as a percentage of a 100% standard (black). The mean intensity of MPO staining per pixel, corrected for background, over all muscle-containing fields was determined in blinded fashion (H-MPW) using ImageJ, thus capturing both the prevalence and intensity of staining per unit area across each muscle.
Statistical Analyses
In vitro experiments were analyzed using two-way analyses of variance (ANOVA) with Tukey’s tests for multiple comparisons. In vivo experiments were subjected to one-way ANOVA followed by Dunnett’s post hoc analysis, comparing each experimental group to PBS-treated controls with ischemia/reperfusion injury. Statistical significance was defined as p < 0.05.
Results
CoPP Induces HO-1 Expression and Activity in MDSCs
A single 24-hour exposure to CoPP increased HO-1 protein expression in MDSCs by 60% compared with vehicle-treated controls (mean relative density 2.42 ± 0.80 vs. 1.51 ± 0.16, mean difference 0.90, 95% confidence interval (CI) of the difference (0.23, 1.57), p = 0.002, Fig. 1A). Without further dosing, elevated expression levels persisted for at least 96 hours (p = 0.003 at 48 hours, p = 0.01 at 96 hours; Fig. 1A). HO-1 enzyme activity, assessed by cGMP, was consistently higher in CoPP-treated cells than in vehicle-only controls at all four time points (p = 0.002 at 48 hours, p = 0.003 at 72 hours; Fig. 1B). The addition of SnPP, a posttranslational steric inhibitor of HO-1 enzyme activity [22], reduced CoPP-induced cGMP to levels comparable to vehicle-only controls (p ≥ 0.89 at all time points).
The graphs illustrate the dynamics of HO-1 expression and activity over time after CoPP exposure. MDSCs were treated for 24 hours with PBS (vehicle only), 25 μM CoPP, or 25 μM CoPP + 25 μM SnPP and then cultured in the absence of CoPP/SnPP over the next 72 hours. Graph (A) shows the time course of HO-1 protein expression relative to β-actin as quantified by Western blot densitometry. Graph (B) shows cGMP production in MDSCs as a measure of HO-1 enzyme activity. Column heights denote mean values with the error bars representing SDs. *p < 0.05; **p ≤ 0.01; ***p < 0.001.
In the hypoxia/reoxygenation experiments, HO-1 protein levels in CoPP-treated MDSCs (mean relative density 2.02 ± 0.60) were double those of vehicle-only controls (0.98 ± 0.11) after 5 hours of hypoxia (mean difference 1.04; 95% confidence interval [CI], 0.50–1.57; p < 0.001; Fig. 2A). After 16 hours of reoxygenation, HO-1 levels in the CoPP-treated group (mean relative density 1.79 ± 0.12) were triple those in either of the two control groups (0.58 ± 0.14 in the vehicle-only group (mean difference 1.21; CI, 0.67–1.75; p < 0.001; 0.60 ± 0.10 in the CoPP + SnPP group, mean difference 1.19; CI, 0.65–1.73; p < 0.001).
The effects of hypoxia/reoxygenation on HO-1 protein levels and apoptosis in MDSCs are shown. Graph (A) presents HO-1 protein levels relative to β-actin quantified by Western blot densitometry. Graph (B) shows apoptotic nuclei as a percentage of total nuclei for the three conditions. Column heights denote mean values and error bars the SDs over all replicates. Section (C) displays representative examples of flow cytometric analyses of propidium iodide-stained MDSCs in several of the replicates to illustrate methodology. The arrows indicate the location of the peaks for the hypodiploid (apoptotic) populations, seen in all treatment groups in the “After Hypoxia” and “Reoxygenation” columns, but present only at negligible levels in the “Before Hypoxia” column. *p < 0.05; ***p < 0.001.
CoPP Exposure Reduces MDSC Apoptosis After Hypoxia/reoxygenation Injury
Vehicle-treated MDSCs subjected to the in vitro hypoxia/reoxygenation protocol exhibited a 20-fold increase in apoptosis at the end of 5 hours of hypoxia (0.8% ± 0.3% apoptotic cells at baseline versus 17.2% ± 0.5% after hypoxia, mean difference 16.4%; CI, 12.9–19.8; p < 0.001). A 70-fold increase over baseline was seen after 16 hours of reoxygenation (57.3% ± 0.5% apoptotic after reoxygenation, mean difference 56.5%; CI, 53.1–59.9; p < 0.001; Fig. 2B). CoPP treatment reduced apoptosis by 17% during hypoxia (14.3% ± 0.9% in the CoPP-treated group versus 17.2% ± 0.5% in vehicle-treated controls after hypoxia, mean difference 2.9%; CI, −0.5 to 6.4; p = 0.14). Cytoprotective effects were most evident after reoxygenation when CoPP-treated MDSCs exhibited a 55% reduction in apoptosis (25.7% ± 0.7% in the CoPP-treated group versus 57.3% ± 0.5% in vehicle-treated controls following reoxygenation, mean difference 31.6%; CI, 28.1–35.0; p < 0.001).
CoPP Reduces Myocyte Damage in Hindlimb Ischemia/reperfusion Injury
Light microscopy of longitudinal sections confirmed that myofibers with EBD inclusion had lost sarcomeres and nuclei, consistent with incipient cell death, whereas unstained cells had maintained their contractile apparatus (Fig. 3A). As quantified by EBD inclusion (Fig. 3A–B), CoPP treatment decreased the extent of myocyte damage in the gastrocnemius. A 38% reduction in myocyte injury was seen when the drug was delivered during ischemia (53.2% ± 14% EBD+ myofibers in the CoPP-treated group versus 86.4% ± 7% in vehicle-treated, injured controls, mean difference 33.2%; 95% CI, 18.3–48.4; p < 0.001). Also in the gastrocnemius, a 30% reduction in injury was seen with drug delivery at the onset of reperfusion (60.6% ± 13% EBD+ with CoPP treatment, mean difference 25.8%; CI, 12.2–39.4; p < 0.001; Fig. 3C). However, in the TA, myocyte damage was decreased only when CoPP was given at the onset of reperfusion, resulting in a 27% reduction in injury (58.3% ± 14% EBD+ myofibers with CoPP treatment versus 78.8% ± 8% in injured controls, mean difference 20.5%; CI, 6.1–35.0; p = 0.004; Fig. 3D). Delaying CoPP delivery until 1 hour after tourniquet release obviated any protective effect in either the gastrocnemius or TA muscles (p = 0.99 and 0.45, respectively). Also, in the group pretreated with the HO-1 inhibitor, SnPP, both the gastrocnemius and TA muscles exhibited muscle damage indistinguishable from vehicle-treated, ischemia/reperfusion-injured controls (p = 0.99 and 0.93, respectively).
Muscle damage after hindlimb ischemia/reperfusion injury was assessed by EBD inclusion. (A) A representative image illustrates EBD staining in a longitudinal section of gastrocnemius muscle from the ischemia/reperfusion-injured control group. Viewed in gray scale, damaged myofibers with intracellular EBD uptake, indicating permeabilized sarcolemmal membranes, are light in color, whereas viable cells that exclude EBD are darker. Scale bar = 100 μm. The magnified inset demonstrates the visible striations in viable myocytes, whereas adjacent cells with EBD inclusion (light) have lost their internal structure. Scale bar = 20 μm. (B) EBD staining is shown in representative cross-sections of ischemia/reperfusion-injured muscle, demonstrating the relative reduction in EBD-positive cells in the CoPP-treated muscles compared with vehicle-treated controls. Scale bar = 500 μm. (C–D) Data points represent the mean EBD-positive area per total analyzed muscle area for each animal. Horizontal lines denote the mean values and SDs for the treatment group. Treatment groups: (A) ischemia/reperfusion injury with PBS injection (vehicle-only control); (B) CoPP delivered during ischemia; (C) CoPP delivered at the onset of reperfusion; (D) CoPP delivered after 1 hour of reperfusion; (E) HO-1 activity inhibitor, SnPP, delivered 24 hours before injury. **p < 0.01; ***p < 0.001.
CoPP Reduces Measures of the Host Inflammatory Response to Ischemia/reperfusion Injury
On histology, leukocyte infiltration, consisting primarily of neutrophils, was evident in PBS-treated, injured muscles after 24 hours of reperfusion (Fig. 4). Notably, leukocyte accumulation was exacerbated in animals pretreated with the HO-1 inhibitor, SnPP. Normal myocyte morphology was relatively preserved in the CoPP-treated group, whereas damaged and degenerating myocytes appeared more prevalent in the PBS-treated controls. Interstitial edema was seen in all limbs sustaining ischemia/reperfusion injury, regardless of treatment group. Uninjured, contralateral limbs in the same animals did not exhibit either edema or leukocyte infiltration.
The photomicrographs illustrate muscle histology after hindlimb ischemia/reperfusion (I/R) injury. Images are representative hematoxylin and eosin-stained sections of injured gastrocnemius and TA muscles from mice in three treatment groups plus a contralateral, uninjured hindlimb. Selected areas have been magnified in the insets. Interstitial edema is observed in all muscles undergoing ischemia/reperfusion injury but is absent in the contralateral leg without direct ischemic injury. Myocyte morphology and peripheral nuclei appear normal in the CoPP-treated group, whereas damaged myocytes are more evident in the PBS-treated, injured control group (TA muscle insets). Leukocyte infiltration appears exacerbated in mice treated with the HO-1 inhibitor, SnPP. Scale bar = 100 μm.
The local inflammatory response to muscle injury was compared between groups by assessing the mean MPO staining intensity across the entirety of each muscle, reflecting neutrophil activation, accumulation, and degranulation (Fig. 5A–B). Reductions in mean MPO immunoreactivity were seen in both the gastrocnemius and TA muscles in treatment groups receiving CoPP compared with vehicle-treated, injured controls. Furthermore, for both muscles, these antiinflammatory effects were observed whether CoPP was delivered during ischemia or at the onset of reperfusion (Fig. 5C–D). Across the gastrocnemius, mean MPO staining intensity was decreased by 28%, from 32.9% ± 9.0% in PBS-treated, injured controls to 23.7% ± 4.2% in groups given CoPP during ischemia (mean difference 9.2%; CI, −1.4 to 19.8; ANOVA p = 0.02, post hoc testing p = 0.10). When CoPP was delivered at the onset of reperfusion, a similar 27% reduction was seen in the gastrocnemius (from 32.9% ± 9.0% to 24.0% ± 9.1%, mean difference 8.9%; CI, −0.8 to 18.6; p = 0.08). In the TA, mean MPO intensity decreased by 34%, from 32.4% ± 5.9% in injured controls to 21.4% ± 3.0% when CoPP was given during ischemia (mean difference 11.1%; CI, 5.0–17.2; p < 0.001). A comparable 31% reduction was also observed in the TA when CoPP was delivered at the onset of reperfusion (to 22.2% ± 3.1%, mean difference versus injured controls 10.3%; CI, 4.5 to 16.0; p < 0.001). However, in both muscles, delaying CoPP delivery until 1 hour after tourniquet release abrogated much of this efficacy (p = 0.45 and 0.89 in the gastrocnemius and TA, respectively). In the groups pretreated with the HO-1 activity inhibitor, SnPP, MPO immunoreactivity was equivalent to or greater than in the PBS-treated, injured controls (p = 0.68 and 0.51 in the gastrocnemius and TA, respectively).
The image and graphs demonstrate MPO immunoreactivity in muscle after ischemia/reperfusion injury. (A) A representative image illustrates MPO immunostaining in a muscle cross-section from the control group after ischemia/reperfusion injury. Arrowheads point to some of the many activated neutrophils that stain positively for MPO (brown chromogen). In this specimen, MPO has also been released into the extracellular space and has permeated some myocytes: (a) indicates a normal myocyte; and (b) a degenerating myocyte with internalized MPO. Scale bar = 100 μm. (B) This higher magnification photomicrograph from another specimen demonstrates damaged cells adjacent to undamaged ones. MPO staining is localized at the cell membranes of damaged cells, with some staining also within these cells. Undamaged cells do not exhibit MPO staining. Scale bar = 100 μm. (C–D) Graphs show the total MPO immunoreactivity in the gastrocnemius and tibialis muscles, respectively. Data points represent the mean MPO staining intensity (as a percent of standard) per unit area of analyzed muscle for each animal. Lower intensity staining reflects lower levels of neutrophil accumulation, activation, and MPO release. Horizontal lines denote mean values and SDs for the treatment group. Treatment groups: (A) ischemia/reperfusion injury with PBS injection (vehicle-only control); (B) CoPP delivered during ischemia; (C) CoPP delivered at the onset of reperfusion; (D) CoPP delivered after 1 hour of reperfusion; (E) HO-1 inhibitor, SnPP, delivered 24 hours before injury. ***p < 0.001.
Discussion
Given the dearth of current treatment options for ischemic limb injury, new therapies to improve skeletal muscle salvage under these conditions could have a considerable impact on functional recovery and patient rehabilitation. HO-1 was targeted in these experiments because its enzymatic endproducts, bilirubin and carbon monoxide, are antiapoptotic, antioxidative, antiinflammatory, and proangiogenic [1, 2, 10]. Additionally, HO-1 activation has been shown to enhance resistance against sepsis [7, 19]. This unique combination of effects would be especially appropriate for trauma care. In these experiments, we examined whether a transcriptional activator of HO-1, CoPP, could have a therapeutic benefit in acute skeletal muscle trauma. The results demonstrated that CoPP induced HO-1 in muscle stem cell populations and, in turn, protected them from apoptosis during in vitro hypoxia/reoxygenation stress. Furthermore, CoPP delivered either during ischemia or at the time of reperfusion reduced both myocyte loss and host inflammatory response in an in vivo model of severe ischemia/reperfusion injury. The therapeutic window was time-dependent, however, because efficacy was lost if treatment was given 1 hour after the start of reperfusion.
Several limitations must be taken into account when interpreting these data. Different doses of CoPP were not tested. Furthermore, evaluation was at 24 hours after reperfusion, by which time over 85% of myocytes already exhibited irreversible damage and many neutrophils had degranulated. Thus, although the consequences of neutrophil infiltration were captured, presaging events occurring in the first hours after injury such as endothelial adhesion molecule expression and neutrophil infiltration were not examined. Both are known to be mitigated by HO-1 in other organs [2, 14, 17, 35]. Also, quantitation of MPO immunohistochemical staining, used to visualize MPO at the site of myocyte damage, is not equivalent to direct measurement of enzyme activity. Finally, although the gastrocnemius exhibited reductions in MPO immunoreactivity at 24 hours, these did not reach statistical significance on post hoc testing. However, both the group receiving CoPP during ischemia (p = 0.10) and CoPP at reperfusion (p = 0.08) showed a statistical trend toward an antiinflammatory effect and, if these two early CoPP therapy arms were combined, then a significant difference was seen between the CoPP-treated groups and the injured controls (ANOVA p < 0.01, Dunnett’s p = 0.03 versus PBS controls). Therefore, we believe the statistical trend toward decreased MPO immunoreactivity in the gastrocnemius, which parallels that seen in the TA, represents a genuine phenomenon.
The first question we addressed was whether HO-1 would be inducible in skeletal muscle, modeled here in MDSCs. MDSCs represent the most primitive and plastic of the muscle progenitors [4, 29]. However, MDSCs, in particular, have a higher baseline antioxidant content than more differentiated cells and are inherently more resistant to oxidative stress [42]. Thus, it was unknown whether HO-1 upregulation would be inducible and functional in MDSCs given that it operates, in part, through antioxidative mechanisms. These in vitro experiments demonstrated that CoPP induction of HO-1 is, indeed, actuated in skeletal MDSCs. Notably, a single exposure to CoPP produced sustained elevations in HO-1 enzyme activity over 4 days, sufficient to cover the acute postinjury period.
Second, we examined whether CoPP exposure would be cytoprotective in MDSCs. Especially where extensive muscle mass has been lost, the regenerative capacity of muscle may depend not only on salvage of existing muscle, but also on preservation of stem cell populations [6]. Importantly, CoPP treatment resulted in a statistically significant 55% reduction in apoptosis for this multipotent stem cell population during simulated ischemia/reperfusion injury.
Third, we asked whether CoPP could limit myocyte damage in vivo and whether treatment initiated after injury would still be efficacious, the key point for translation to trauma care. To answer this, we varied the timing of CoPP delivery to reflect realistic clinical scenarios: (1) drug delivery during ischemia, simulating administration by paramedics in the field; (2) delivery at the onset of reperfusion, modeling restitution of blood flow (or tourniquet removal) on patient arrival at the hospital or during trauma surgery; and (3) delayed delivery 1 hour after the start of reperfusion to determine the therapeutic window. A model of severe injury was used to rigorously test efficacy under conditions simulating combat casualties or other major trauma. Moreover, the mechanism of injury in this in vivo model was different than in vitro with necrosis predominating over apoptosis. However, even under these adverse conditions, CoPP was found to reduce myofiber damage when delivered after injury.
Responsiveness to CoPP, however, varied between the gastrocnemius and the TA muscles. Whereas in the gastrocnemius, muscle damage could be ameliorated by giving CoPP either during ischemia or at the onset of reperfusion, in the TA muscle, CoPP delivery was effective only at the onset of reperfusion. Differences in fiber type composition between the two muscles are unlikely to be solely responsible for this discrepancy. Although fast-twitch fibers have been reported to be more susceptible to ischemic injury [5, 43], both of these muscles are predominantly composed of fast-twitch fibers in mice. Instead, it seems more likely that the TA muscle, located within the anterior compartment, may have been compromised by emergent compartment syndrome. Although this ischemia/reperfusion model was not one of compartment syndrome, per se, some degree of the syndrome may well have been produced under these experimental conditions given the edema documented on histology. Thus, in the closed anterior compartment, pressure phenomena may have contributed to the TA being more sensitive to oxidative injury and more resistant to therapeutic interventions, especially those treatments initiated during ischemia in the absence of blood flow.
From a clinical perspective, the most noteworthy finding in these in vivo experiments was that CoPP delivery at the time of reperfusion (tourniquet release) was still effective at reducing myocyte injury even in the treatment-refractory anterior compartment. Thus, CoPP could hold promise as a much-needed new therapy for impending or suspected compartment syndrome, a potential that would warrant further investigation. Supporting this possibility is a new report showing that, in rats, systemic treatment with novel molecules that release carbon monoxide, a direct product of HO-1 enzymatic activity, can diminish microvascular dysfunction and tissue injury in compartment syndrome [16].
The final study question was whether the effects of CoPP on muscle preservation might be attributable, at least in part, to its immunomodulatory effects, evidenced by reductions in MPO immunoreactivity. In these experiments, CoPP was found to decrease the intensity and prevalence of MPO immunoreactivity whether the drug was given during ischemia or at the time of reperfusion. MPO, released by neutrophil degranulation, interacts with locally generated superoxides and hydrogen peroxide to produce hypochlorous acid and other cytotoxic reactants. These not only directly cause myocyte membrane lysis and cell death [39, 41], but also contribute to the secondary systemic effects of traumatic injury on distant organs such as acute respiratory distress syndrome [9, 27]. MPO-related cytotoxicity is influenced by the oxidative/nitrosative state of the tissue environment [39], which can be modulated by HO-1 through its potent antioxidant effects, catabolism of prooxidant free heme, and stabilization of mitochondrial function [2, 10].
Looking toward clinical translation, although CoPP has been proposed for clinical use [33], it is not FDA-approved. To expedite transition to the clinic, drugs in current use that are known to activate the nrf2/HO-1 pathway [1], although with less specificity, might be substituted for CoPP in future trials. Examples would include the statins and the phosphodiesterase-5 inhibitors. Of interest, hyperbaric oxygen therapy, already approved for use in acute traumatic injuries, and known to reduce neutrophil adhesion in experimental skeletal muscle ischemia [45], has now been shown to induce nrf2/HO-1 in ischemic cardiac muscle as its mechanism of myocardial protection [44]. Thus, hyperbaric oxygen could be an alternate means to upregulate HO-1 in limb ischemia, although its effects on oxygen radicals in this setting would require evaluation.
Taken together, these results demonstrate that CoPP, or possibly other substitute nrf2/HO-1 inducers, have potential to be used clinically to enhance the salvage of muscle mass and muscle progenitor cells in ischemic skeletal muscle injury, offering a new solution to a problem for which there are currently few therapeutic options. Such a single-dose drug or treatment that can be easily delivered in the field, on arrival at the hospital, or during trauma surgery, and that offers extended activity over several days, would be readily adaptable to the medical situations faced on the battlefield, during extended medical evacuations, and at regional trauma centers. Beyond the critical care setting, these results also suggest that HO-1 induction in MDSCs and other muscle precursors could be used to improve the survival of implanted cells in cellular therapies for tissue regeneration and limb reconstruction.
References
Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127.
Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279.
Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–630.
Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, Cummins J, Epperly M, Qu-Petersen Z, Huard J. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–646.
Chan RK, Austen WG Jr, Ibrahim S, Ding GY, Verna N, Hechtman HB, Moore FD Jr. Reperfusion injury to skeletal muscle affects primarily type II muscle fibers. J Surg Res. 2004;122:54–60.
Chen XK, Rathbone CR, Walters TJ. Treatment of tourniquet-induced ischemia reperfusion injury with muscle progenitor cells. J Surg Res. 2011;170:e65–73.
Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247.
Crawford RS, Hashmi FF, Jones JE, Albadawi H, McCormack M, Eberlin K, Entabi F, Atkins MD, Conrad MF, Austen WG Jr, Watkins MT. A novel model of acute murine hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H830–837.
Eliason JL, Wakefield TW. Metabolic consequences of acute limb ischemia and their clinical implications. Semin Vasc Surg. 2009;22:29–33.
Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354.
Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans blue dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69–79.
Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, Southard D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287–292.
Kawamoto S, Flynn JP, Shi Q, Sakr SW, Luo J, Allen MD. Heme oxygenase-1 induction enhances cell survival and restores contractility to unvascularized three-dimensional adult cardiomyocyte grafts implanted in vivo. Tissue Eng Part A. 2011;17:1605–1614.
Konrad FM, Braun S, Ngamsri KC, Vollmer I, Reutershan J. Heme oxygenase-1 attenuates acute pulmonary inflammation by decreasing the release of segmented neutrophils from the bone marrow. Am J Physiol Lung Cell Mol Physiol. 2014;307:L707–717.
Lakkisto P, Siren JM, Kyto V, Forsten H, Laine M, Pulkki K, Tikkanen I. Heme oxygenase-1 induction protects the heart and modulates cellular and extracellular remodelling after myocardial infarction in rats. Exp Biol Med (Maywood). 2011;236:1437–1448.
Lawendy AR, Bihari A, Sanders DW, Potter RF, Cepinskas G. The severity of microvascular dysfunction due to compartment syndrome is diminished by the systemic application of CO-releasing molecule (CORM-3). J Orthop Trauma. 2014;28:e263–268.
Lin YT, Chen YH, Yang YH, Jao HC, Abiko Y, Yokoyama K, Hsu C. Heme oxygenase-1 suppresses the infiltration of neutrophils in rat liver during sepsis through inactivation of p38 MAPK. Shock. 2010;34:615–621.
Luo J, Weaver MS, Cao B, Dennis JE, Van Biber B, Laflamme MA, Allen MD. Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo. Stem Cells Trans Med. 2014;3:734–744.
MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, Piantadosi CA. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2012;185:851–861.
McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004;561:233–244.
Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell’Acqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607.
Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475–1479.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136.
Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA 3rd, Kukreja RC. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–548.
Pachori AS, Melo LG, Hart ML, Noiseux N, Zhang L, Morello F, Solomon SD, Stahl GL, Pratt RE, Dzau VJ. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc Natl Acad Sci U S A. 2004;101:12282–12287.
Park JJ, Campbell KA, Mercuri JJ, Tejwani NC. Updates in the management of orthopedic soft-tissue injuries associated with lower extremity trauma. Am J Orthop. 2012;41:E27–35.
Peng TC, Jan WC, Tsai PS, Huang CJ. Heme oxygenase-1 mediates the protective effects of ischemic preconditioning on mitigating lung injury induced by lower limb ischemia-reperfusion in rats. J Surg Res. 2011;167:e245–253.
Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–814.
Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864.
Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461.
Saxena S, Shukla D, Saxena S, Khan YA, Singh M, Bansal A, Sairam M, Jain SK. Hypoxia preconditioning by cobalt chloride enhances endurance performance and protects skeletal muscles from exercise-induced oxidative damage in rats. Acta Physiol. 2010;200:249–263.
Scalea TM, DuBose J, Moore EE, West M, Moore FA, McIntyre R, Cocanour C, Davis J, Ochsner MG, Feliciano D. Western Trauma Association critical decisions in trauma: management of the mangled extremity. J Trauma Acute Care Surg. 2012;72:86–93.
Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. Faseb J. 2006;20:2651–2653.
Smith JK, Grisham MB, Granger DN, Korthuis RJ. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol. 1989;256:H789–793.
Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563.
Stansbury LG, Lalliss SJ, Branstetter JG, Bagg MR, Holcomb JB. Amputations in US military personnel in the current conflicts in Afghanistan and Iraq. J Orthop Trauma. 2008;22:43–46.
Stein AB, Tang XL, Guo Y, Xuan YT, Dawn B, Bolli R. Delayed adaptation of the heart to stress: late preconditioning. Stroke. 2004;35:2676–2679.
Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–385.
Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–353.
Tongers J, Knapp JM, Korf M, Kempf T, Limbourg A, Limbourg FP, Li Z, Fraccarollo D, Bauersachs J, Han X, Drexler H, Fiedler B, Wollert KC. Haeme oxygenase promotes progenitor cell mobilization, neovascularization, and functional recovery after critical hindlimb ischaemia in mice. Cardiovasc Res. 2008;78:294–300.
Tran TP, Tu H, Liu J, Muelleman RL, Li YL. Mitochondria-derived superoxide links to tourniquet-induced apoptosis in mouse skeletal muscle. PLoS One. 2012;7:e43410.
Urish KL, Vella JB, Okada M, Deasy BM, Tobita K, Keller BB, Cao B, Piganelli JD, Huard J. Antioxidant levels represent a major determinant in the regenerative capacity of muscle stem cells. Mol Biol Cell. 2009;20:509–520.
Walters TJ, Kragh JF, Baer DG. Influence of fiber-type composition on recovery from tourniquet-induced skeletal muscle ischemia-reperfusion injury. Appl Physiol Nutr Metab. 2008;33:272–281.
Yin X, Wang X, Fan Z, Peng C, Ren Z, Huang L, Liu Z, Zhao K. Hyperbaric oxygen preconditioning attenuates myocardium ischemia-reperfusion injury through upregulation of heme oxygenase 1 expression: PI3K/Akt/Nrf2 pathway involved. J Cardiovasc Pharmacol Ther. 2015 Jan 20 [Epub ahead of print].
Zamboni WA, Roth AC, Russell RC, Graham B, Suchy H, Kucan JO. Morphologic analysis of the microcirculation during reperfusion of ischemic skeletal muscle and the effect of hyperbaric oxygen. Plast Reconstr Surg. 1993;91:1110–1123.
Zheng J, Wang R, Zambraski E, Wu D, Jacobson KA, Liang BT. Protective roles of adenosine A1, A2A, and A3 receptors in skeletal muscle ischemia and reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;293:H3685–3691.
Acknowledgments
We thank Pamela Y. Johnson PhD, and Mary Beauchamp for tissue sectioning and staining; Leonard D’Amico PhD, and Matthew S. Weaver PhD, for technical assistance with the surgical procedures; Johnny Huard PhD, and his laboratory for technical guidance on MDSC isolation and culture; and Virginia M. Green PhD, for reviewing and editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was funded by USAMRMC Award W81XWH-07-01-0246 (MDA) and USAMRMC Award W81XWH-10-01-0789 (MDA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Benaroya Research Institute, Seattle, WA, USA.
The first three authors contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11999_2015_4332_MOESM1_ESM.tif
Supplementary Fig. 1 Laser Doppler perfusion imaging demonstrates the equivalent efficacy of hindlimb blood flow occlusion by tourniquet application versus surgical femoral artery ligation in two C57BL/6J mice. The tourniquet or arterial suture ligature was applied to the left hindlimb (lower leg in each photo) for 2 hours and then released with recovery of blood flow. The contralateral hindlimb (upper leg in each photo) and tail had no intervention. Blood perfusion is depicted on a color scale from blue to red with red indicating greater flow. Supplementary material 1 (TIFF 5513 kb)
About this article
Cite this article
Wilson, HM.P., Welikson, R.E., Luo, J. et al. Can Cytoprotective Cobalt Protoporphyrin Protect Skeletal Muscle and Muscle-derived Stem Cells From Ischemic Injury?. Clin Orthop Relat Res 473, 2908–2919 (2015). https://doi.org/10.1007/s11999-015-4332-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-015-4332-8