Abstract
Nowadays, coating systems have to fulfill a wide range of requirements. In addition to mechanical properties such as hardness and elasticity, resistance and weatherability, specifically corrosion or chemical resistance are also important. Increasing attention is also being paid to points such as the use of sustainable reactants or the energy optimization of synthesis processes.1 The use of enzymes in the synthetic processes offers two main advantages: firstly, reaction temperatures can be significantly reduced, for example in the production of polyesters, and as a result and a major advantage, certain functional groups can be selectively retained during the reaction.2,3 Thus, for example, aromatic hydroxyl groups can be obtained, while aliphatic groups are esterified.4,5 This allows the preparation of polyesters that do not only have terminal OH groups, but hydroxyl groups within the chain that can act as additional crosslinking points during network formation or as adhesion-promoting groups.6,7 In this work, the influence of such an aliphatic–aromatic polyester, produced enzymatically at low temperatures, on the coating properties is investigated when using different hardener components. Coating formulations were created, and the required OH functionality and the hydroxyl number of the enzymatic polyester have been calculated by using two different, independent methods. Besides the development of guide formulations, the unique mechanical properties of coatings based on the enzymatic polyester were studied. In addition to comparative analysis of network densities, the coatings were also investigated by IR spectroscopy in order to assess the network formation reaction spectroscopically. It can be shown that additional OH groups in the polyester chain increase the network density, but this is not at the expense of elasticity. Thus, enzymatically produced polyesters combine the advantages of low reaction temperatures during production with a unique property profile due to aliphatic and aromatic moieties as well as the partial preservation of OH groups within the chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroxy-functionalized polyesters, crosslinked by polyisocyanates, melamines or epoxides, have been used in the coating industry for decades. The production of the polyester requires high reaction temperatures of 150–280°C and often leads to undesirable side reactions.8,9,10 Furthermore, metal catalysts based on zinc, antimony or cobalt are used, which limit the selective conversion of higher functional monomers at high temperatures and therefore lead to the formation of undesired branched polyesters.11,12,13,14
The enzymatically catalyzed production of hydroxy-functional linear polyesters based on lipase catalysts offers the advantage of very mild reaction temperatures of 50°C, in addition to a biodegradable catalyst, which is biodegradable and does not remain in the resin after synthesis.9
What is particularly interesting is the fact that lipases have individual regioselectivities that allow certain reactive functional groups not to be affected during the reaction. This possibility would only exist performing complex protection and deprotection steps when synthesizing polyesters in form of conventional thermic polycondensation.15
Thus, when using enzymatically catalyzed synthesis, additional aromatic hydroxyl groups can be implemented in the polyester chain, which on the one hand have an adhesion-promoting effect and on the other hand can represent further crosslinking points during crosslinking with the hardener component. In particular, the combination of aliphatic and aromatic monomers in one polyester leads to excellent physical and mechanical properties and a favorable cost structure.16,17
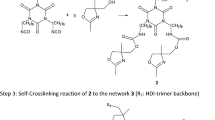
In the present work, the polyester based on adipic acid, 1,6-hexanediol and 2,6-bis (hydroxymethyl) -p-cresol, first introduced in the publication by Seithümmer et al.,18 has been applied in developed coating formulations with different hardener components, such as polyisocyanates, epoxy and melamine building blocks. Therefore, the OH functionality and OH number were calculated using different, independent methods. Furthermore, application and mechanical properties of the enzymatically produced polyester were compared with two commercially available, short-chain polyesters (Desmophen® VP LS 2328 and Desmophen® 800). Desmophen® VP LS 2328 is a linear polyester, while Desmophen® 800 is highly branched and has a high OH-content.
The schematic structure of the polyester produced enzymatically at 50°C prior to chain extension via transesterification with glycerol is shown in Fig. 1.
After the formation of the oligoester, there are still terminal acid groups, which are reacted with glycerol in a second step and lead to chain elongation or, in the case of a simple reaction, to a further increase in OH functionality.
The polyester obtained by chain elongation with glycerol has been used for this work.
Due to the linear aliphatic and aromatic structure with additional hydroxyl groups located within the polyester chain, the coating films are expected to have excellent mechanical properties. The presence of aromatic constituents and two types of hydroxy groups allows the expectation of a high film hardness with simultaneous elasticity and good surface adhesion. Furthermore, when applying the synthesized polyester, it is expected to achieve higher crosslinking density and better chemical resistance of the coating in comparison with other tested polyesters.19,20
Raw materials
Short chained polyesters Desmophen® VP LS 2328 and Desmophen® 800 as well as isocyanate crosslinker Desmodur® ultra N 3600 were generous gifts from Covestro AG (Leverkusen, Germany). The aliphatic polyfunctional epoxy resin based on glycerol, ipox® CL 12, was kindly provided by ipox-chemicals (Laupheim, Germany). The isobutylated melamine-formaldehyde resin Maprenal® MF 800/55iB was kindly provided by BYK-Chemie (Wesel, Germany). The used catalysts such as p-toluenesulfonic acid (PTSA), 1,4-diazabicyclo[2.2.2]octane (DABCO), dibutyltin dilaurate (DBTDL), and all solvents were purchased from Sigma-Aldrich and are used without further purification.
The following tables show the properties of the binders, and crosslinkers on the basis of which the crosslinking ratio and formulations have been calculated:
Analytical methods
The hydroxyl number has been determined by DIN EN ISO 4629-2:2016 three times. For calculation, the average number has been used.
IR spectra, measured on a Bruker Lumos FTIR-Microscope with a resolution of 4 cm−1, are baseline corrected and normalized. For normalization, a signal is used whose intensity does not change during the crosslinking reaction. Depending on the binder system, different bands were used, which are indicated in each case in the spectrum evaluation.
The pendulum damping values have been obtained according to DIN EN ISO 1522 using a Königspendel from Erichsen. Three measurements for each panel were performed 4 h after baking, and average values are given.
The adhesion of the coating films was determined by performing the cross-cut test according to DIN EN ISO 2409 after application on steel panels. Furthermore, the resistance of the samples in the event of sudden deformation based on DIN EN ISO 6272-1:2011 has been determined. For the evaluation, a drop height of 1 m was chosen and it was judged as to whether the film cracked and if it still adhered to the steel surface after the indirect impact of the 1-kg weight.
The contact angle is measured three times with water as testing substance according to DIN EN ISO 19403-2:2020 with the OCA15 measuring device from Dataphysics Instruments. Average values of three measurements are given.
To assess the chemical resistance of the coating films, one drop of different chemicals has been put on the film surface, absorbed and wiped away after 1 min using light pressure with a piece of cloth. Then, the treated area of the coating film is compared to the untreated one and the impact of the chemical on the coating film is visually judged. The value of one means that there was no impact of the chemical on the coating film; a value of six means that the coating film is completely peeled off. For the experiments, methyl ethyl ketone (MEK), ethanol, acetone, water, and dimethyl sulfoxide (DMSO) have been used.
Furthermore, sol–gel analysis has been performed to draw conclusions on the network density of the different polyester coating films. For this, the polymer networks are extracted with MEK in a Soxhlet extractor for 16 h. A double determination is carried out, and the mean value is given (Tables 1 and 2).
Application testing
In order to comparatively test the application properties of different polyesters, the weights were selected in such a way that the reaction of the functional groups of the polyester with the respective hardener component is equimolar.
The solvents were selected, on the one hand, according to their compatibility with the coating system and, on the other hand, on the basis of their boiling ranges, so that uniform oven drying and crosslinking can be achieved. Preliminary tests were carried out to ensure this. The use of surface-active additives, as used in coating formulations, has been deliberately avoided, so that any interaction between additives and binder or hardener component can be ruled out.
Table 3 shows all formulations of the different polyesters with the hardener components, including solvents and catalysts. Weights are given in grams.
Annotation
Sample number 4 (Desmophen® 800 and Desmodur® ultra N 3660) is crosslinked with a 20% excess of Desmophen® 800 because 1:1 crosslinking leads to a coating film with too high crosslinking-density, which does not have any surface adhesion.
Sample number 8 (Desmophen® VP LS 2328and ipox® CL 12) does not lead to a crosslinked polymer network. Higher baking temperatures of 160 °C, doubling the amount of catalyst or working with a 30% higher amount of epoxide, do not lead to crosslinked coating films.
All samples were applied on cleaned steel panels (type DX51D+Z) by a squeegee and baked for 30 min in the oven at a temperature of 140°C to achieve a dry film thickness of 40 µm. The mean values of the coating thicknesses of the individual samples are presented in tabular form in the supplementary information, Table S1.
Results and discussion
The value of OH functionality has been calculated in two different, independent ways. NMR-spectroscopy allows to determine the percentage incorporation of hydroxyl-functional monomers into the chain of the polymer before post-polymerization with glycerol. The molar ratio of 1,6-hexanediol and 2,6-bis (hydroxymethyl)-p-cresol in the chain is 0.38 to 0.62.18 Considering acid numbers decreasing from a theoretical value of 375 mg KOH/g to a value of 73 mg KOH/g during the reaction, around 20% of the polymer chains are acid functional before linking with glycerol. Because the molar composition and the initial weight are known, the mean functionality without additional aromatic OH-groups of the polyester can be calculated and has a value of FOH-theo. terminal = 1.80 (F = FOH-theo. terminal − Facid.funct. = 2.00–0.20).10 By using acid number and 1H-NMR data of the oligoester of Seithümmer et al.,18 the molar composition of the enzymatic polyester before chain lengthening can be determined (Table 4).
Furthermore, an equivalent weight for the enzymatically unaffected aromatic hydroxyl groups of incorporated 2,6-bis (hydroxymethyl)-p-cresol can be determined: Equivalent weightaromat. OH = M2,6-bis (hydroxymethyl)-p-cresol = 168.19 g/mol. Since, according to Table 3, only 31% of the polyester consists of the cresol component, the aromatic OH equivalent weight of the polymer is 168.19 g/mol: 0.31 mol% = 542 g/mol aromatic hydroxyl groups.
Using number average molecular weight Mn = 2400 g/mol from 1H-NMR end group analysis of oligoester,18 FOH = 4.43 (Mn/OH equivalent weight of the polymer) aromatic OH-functionalities are added to the chain enzymatically without further reaction. When the terminal FOH-theo.terminal = 1.80 OH groups are added, the calculated hydroxyl functionality of the oligoester before chain extension is FOH = 6.23.
After post-polymerization with an excess of glycerol (10:1),18 the acid number decreased to a value of 33 mg KOH/g. Since a strong glycerol excess has been used, it can be assumed that mainly a simple addition of glycerol takes place instead of a chain extension and thus, the average OH functionality of the enzymatically synthesized polymer is increased by about FOH = 1.1. Thus, the calculated functionality of the polyester using acid number and 1H-NMR data is approximately FOH = 7.2.
The OH number of 168 mg KOH/g titrated according to DIN EN ISO 4629-2:2016 of the extended polymer can be converted into an OH equivalent weight and is 334 g/mol OH.19 By using the number average molecular weight of the chain-extended polymer, obtained by GPC analysis, of Mn = 2760 ± 140 g/mol,18 the functionality can also be calculated by dividing Mn by the equivalent weight. Thus, an OH-functionality between FOH = 7.8–8.7 is obtained.
Since the numerical values determined by GPC (Mn = 2760 ± 140 g/mol) and NMR (Mn = 2800 g/mol) are highly correlated, the OH functionality of FOH = 7.8–8.6, which is very high for a nearly linear polyester, can be confirmed by two independent wet chemical methods, respectively. On the one hand, the acid number values were used in combination with 1H-NMR data, and on the other hand, the hydroxyl number values were used with those of the GPC data, with both methods yielding similar results.
For the calculations of the formulations, the minimum hydroxyl functionality of FOH = 7.2 has been used, since small amounts of unreacted OH groups do not adversely affect the coating films after crosslinking.
IR spectroscopy is used to confirm the crosslinking reaction of the coatings after baking and to elucidate the polymer network structure. Figure 2 shows the IR spectra of the polyesters crosslinked with the epoxy resin after curing.
IR (resonance in [cm−1] and type): 3300–3500 (OH valence), 2930 (CH3 stretch), 2870 (CH2 sym. stretch), 1730 amide I (C=O stretch), 1607 (C=C conj. aromat.), 1465 (CH2 deformation), 1260 (OH deformation), 1260–1240 (C-O stretch epoxide),
1250 (=C-O-C), 1130, 1080 (C–O–C valence), 863 (aromatic CH out of plane), 750 (C=O out of plane), spectra not normalized due to lack of possible normalization peak.21,22,23
After crosslinking with the epoxy resin ipox® CL 12, high similarities are present in the IR spectra. Characteristic ether valence vibrations are present at 1130 cm−1 and 1080 cm−1. Furthermore, OH groups formed by ring opening are visible at 3500–3300 cm−1 and at 1260 cm−1 (OH deformation) for both polyesters. Characteristic epoxide vibrations, for example C-O stretch epoxide at 1280–1230 cm−1, are superimposed, so that no reliable statement can be made as to whether the ring opening of the glycidyl groups has occurred completely. In the paint film of Desmophen® 800, the C–O stretch vibration of the oxirane ring at 840 cm−1 indicates that the reaction has not been completed. Furthermore, the aromatic vibrations specific to the enzymatic polyester are present at 1607 cm−1 (C=C conj. aromat.) and 863 cm−1 (aromatic CH out of plane). Both IR spectra suggest that many OH groups remain after crosslinking, so that very soft, but crosslinked, coating films can be expected (Figs. 3, 4).
Enlargement of Fig. 3 from 1200 to 1800 cm−1
IR (resonance in [cm−1] and type): 3350 (NH–COO and NH stretch), 2997 (CH3 stretch), 2920 (CH2 stretch), 1715 (C=O, urethane), 1685 amide I (C=O, urethane, isocyanurate), 1615 (C=C conj. aromat.), 1530 amide II (NH–COO and NH bending), 1460 (CH3 deformation asym.), 1240 (=C-O-C, urethane), 1175, 1140 (C–O–C valence), 863 (aromatic CH out of plane), 765 (C–N stretch, isocyanurate, normalization peak).23,24,25
Finally, the reactions of the different polyesters with the HDI-trimer Desmodur® ultra N 3600 also show high similarities and the urethane bands formed via successful crosslinking, for example the urethane/amide I band, can be detected at 1715 cm−1 and 1685 cm−1 with the corresponding amide II or N–H signal at 1530 cm−1 caused by N–H-bending vibration of the urethane groups. An amide II signal is visible, if at least one proton is bound to the nitrogen of the amide.26 Furthermore, C–O–C-valence of the urethane peaks at 1175 cm−1 and 1140 cm−1 is visible.
The coating film in which the enzymatic polyester has been used exhibits the characteristic aromatic vibrations of cresol at 1615 cm−1 (C=C conj.aromat.) and 863 cm−1 (aromatic CH out of plane).23,27 Thus, it is expected that all urethane-based coating films are crosslinked and have similar application properties.
Figure 5 represents the results of the crosslinking reactions of the polyesters with the melamine Maprenal® MF 800/55iB resin:
IR (resonance in [cm−1] and type): 3350 (NH–COO and NH stretch), 2960 (CH3 stretch), 2865 (CH2 stretch), 1715 (C=O stretch, 1650 (C=C conj. aromat.), 1530 (NH bending, ring stretch triazine), 1470 (CH deformation asym.), 1360 (ring stretch triazine), 1255 (=C–O–C), 1165, 1140 (C–O–C valence), 863 (aromatic CH out of plane), 813 (sym. out of plane vibration, triazine, normalization peak).23,28,29
Here, very similar IR spectra can be found for the different polyesters being crosslinked with the melamine resin. It is striking that NH bands at 3350 and 1530 cm−1 are present in all films, although a complete butylated melamine resin has been used. Wysoglad29 shows in his thesis that the butylation of melamine resins is incomplete and thus, NH groups are still present after crosslinking.
The presence of C-O-C ether vibrations at 1165 and 1140 cm−1 in all spectra indicates that the crosslinking reaction has been successfully carried out. Furthermore, specific triazine bands are present at 1530 and at 813 cm−1.23,30 Again, in the spectrum of the enzymatic polyester coating the specific peaks of the aromatic ring are found at ca. 1650 cm−1 (C=C conj. aromat.) and 863 cm−1 (aromatic CH out of plane).
After spectroscopic observation of the coating films and confirming successful crosslinking reaction, application tests are carried out. Results are presented in Tables 5 and 6.
When crosslinked with the melamine resin Maprenal® MF 800/55iB, the coating film with the enzymatic polyester has by far the highest film hardness (110 enzymatic, 37 star-polyester and 25 linear polyester). The chemical resistance of the coating film containing the enzymatic polyester is over all tested substances a little higher to comparative polyesters. Here, the chemical resistances of coatings containing enzymatic polyester exceed the performance of formulations containing the branched polyester Desmophen® 800. When crosslinked with the Maprenal® MF 800 / 55iB, the coating film made from the enzymatically synthesized polyester shows a slight incompatibility, which can possibly be attributed to the higher hydrophobicity of the enzymatic polyester, which also explains the poorer adhesion to the surface and the slight clouding of the coating film. However, the measured contact angles do not show this phenomenon, as similar values to the comparison polyesters have been determined. Thus, the aromatic-aliphatic polyester is only partly suitable for use with a melamine resin.
The use of the glycerol polyglycidyl ether ipox® CL 12 as a crosslinking component for the polyester leads to very soft, sometimes tacky films. The film hardness when using the short-chain, linear polyester Desmophen® VP LS 2328, is not sufficient for performing application tests. It is interesting here that the enzymatically produced polyester has an approximately 15% higher gel content than the star polyester Desmophen® 800, a higher film hardness with higher elasticity and chemical resistance at the same time. When performing contact-angle test, the water drop on sample including Desmophen® 800 spreads immediately and an angle of 70.5° has been determined while for the coating made from the enzymatic polyester, the angle is 83.6°. Although the enzymatic polyester has a linear structure, the mechanical properties are consistently better compared to the branched polyester Desmophen® 800. This illustrates the positive effect of the linear structure with additional hydroxyl groups within the chain, as the chain mobility is higher compared to a branched polyester. Only the yellow coloration of the coating film containing the enzymatic polyester is to be assessed as disadvantageous.
The highest performance of the enzymatic polyester can be observed when the isocyanate hardener Desmodur® ultra N 3600 is used for crosslinking. With an average of 133 pendulum swings, the film hardness is only slightly higher than that of the branched polyester Desmophen® 800 with a value of 125, but the film elasticity and surface adhesion of the linear, enzymatically produced polyester are significantly higher than that of Desmophen® 800. For example, the enzymatic polyester achieves the best values in the cross-cut test and in the impact test, while the branched polyester splinters in both tests—despite a 1.2-fold excess of polyester being used—no longer adhere to the metal surface after the tests. Figure S2, presented in the supplementary information, pictures the results of the cross-cut and impact test of both polyesters in direct comparison.
The coating film with the linear, dihydroxy-functional polyester Desmophen® VP LS 2328 also has high flexibility and good surface adhesion, but is much softer (25 pendulum swings), and the chemical resistance and the gel content are lower compared to the other polyesters. This shows that the enzymatically produced polyester, especially when used in combination with the hexamethylene diisocyanate trimer Desmodur® ultra N 3600, combines the properties of a linear polyester with high elasticity and good adhesion with those of a highly branched polyester such as Desmophen® 800 with high hardness, increased network density and chemical resistance.
A visual assessment of the various coating films is presented together with pictures in the supplementary information, Figs. S3-S5.
Further advantages are the very low reaction temperatures of 50°C while synthesizing the enzymatic polyester and the lack of metal catalysis in the synthesis. The regioselective lipase B from Candida antarctica (CAL-B) used for catalysis can also be used multiple times.3 The synthesis of the enzymatic polyester-polyol in an industrial scale poses a challenge. Nevertheless, the property profile is unique due to the high gel content and chemical resistance, and in particular, the combination of high film hardness with simultaneous elasticity and surface adhesion illustrates the advantages of a linear polyester with additional, secondary hydroxyl groups within the chain.
The enzymatically produced polyester can be used particularly in application fields in which high mechanical demands are made with regard to the mechanical properties and a slight yellowing of the coating is not to be assessed as disadvantageous.
Conclusions
In this work, the high hydroxyl functionality of the enzymatically prepared aromatic-aliphatic linear polyester was confirmed by two independent measurement methods and based on these results paint formulations were calculated. Particularly when used in the tested polyisocyanate and epoxy resin systems, the enzymatically synthesized polyester exhibits very good application properties, since contrary properties such as hardness and flexibility are combined by the linear structure with additional hydroxyl groups. Thus, the polymer networks are highly branched and have good adhesion, but are not brittle, so that the polyester can be used primarily in areas with high mechanical requirements without great decorative demands.
References
Goldschmidt, A, Streitberger, HJ, BASF Handbook on Basics of Coating Technology, 2., rev. ed.; Vincentz Network: Hannover (2007)
Pellis, A, Herrero Acero, E, Ferrario, V, Ribitsch, D, Guebitz, GM, Gardossi, L, “The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters.” Trends Biotechnol., 34 316–328. https://doi.org/10.1016/j.tibtech.2015.12.009 (2016)
Kundys, A, Białecka-Florjańczyk, E, Fabiszewska, A, Małajowicz, J, “Candida antarctica Lipase B as Catalyst for Cyclic Esters Synthesis, Their Polymerization and Degradation of Aliphatic Polyesters.” J. Polym. Environ., 26 396–407. https://doi.org/10.1007/s10924-017-0945-1 (2018)
Li, G, Yao, D, Zong, M, “Lipase-Catalyzed Synthesis of Biodegradable Copolymer Containing Malic Acid Units in Solvent-Free System.” Eur. Polym. J., 44 1123–1129. https://doi.org/10.1016/j.eurpolymj.2008.01.027 (2008)
Yao, D, Li, G, Kuila, T, Li, P, Kim, NH, Kim, S-I, Lee, JH, “Lipase-Catalyzed Synthesis and Characterization of Biodegradable Polyester Containing l-Malic Acid Unit in Solvent System.” J. Appl. Polym. Sci., 120 1114–1120. https://doi.org/10.1002/app.33257 (2011)
Organic Coatings: Science and Technology, 3rd edn. Wiley, Hoboken (2007)
Nakamura, S, Tsuji, Y, Yoshizawa, K, “Role of Hydrogen-Bonding and OH-π Interactions in the Adhesion of Epoxy Resin on Hydrophilic Surfaces.” ACS Omega, 5 26211–26219. https://doi.org/10.1021/acsomega.0c03798 (2020)
Fodor, C, Golkaram, M, Woortman, AJJ, van Dijken, J, Loos, K, “Enzymatic Approach for the Synthesis of Biobased Aromatic–Aliphatic Oligo-/Polyesters.” Polym. Chem., 8 6795–6805. https://doi.org/10.1039/C7PY01559C (2017)
Hevilla, V, Sonseca, A, Echeverría, C, Muñoz-Bonilla, A, Fernández-García, M, “Enzymatic Synthesis of Polyesters and their Bioapplications: Recent Advances and Perspectives.” Macromol. Biosci., 21 e2100156. https://doi.org/10.1002/mabi.202100156 (2021)
Poth, U. Polyester und Alkydharze: Grundlagen und Anwendungen. Vincentz Network, Hannover (2005)
Egorova, KS, Ananikov, VP, “Toxicity of Metal Compounds: Knowledge and Myths.” Organometallics, 36 4071–4090. https://doi.org/10.1021/acs.organomet.7b00605 (2017)
Greimel, KJ, Perz, V, Koren, K, Feola, R, Temel, A, Sohar, C, Herrero Acero, E, Klimant, I, Guebitz, GM, “Banning Toxic Heavy-Metal Catalysts from Paints: Enzymatic Cross-Linking of Alkyd Resins.” Green Chem., 15 381. https://doi.org/10.1039/c2gc36666e (2013)
Cameron, DJA, Shaver, MP, “Aliphatic Polyester Polymer Stars: Synthesis, Properties and Applications in Biomedicine and Nanotechnology.” Chem. Soc. Rev., 40 1761–1776. https://doi.org/10.1039/c0cs00091d (2011)
Schallausky, F, Erber, M, Komber, H, Lederer, A, “An Easy Strategy for the Synthesis of Well-Defined Aliphatic-Aromatic Hyperbranched Polyesters.” Macromol. Chem. Phys., 209 2331–2338. https://doi.org/10.1002/macp.200800346 (2008)
You, Z, Cao, H, Gao, J, Shin, PH, Day, BW, Wang, Y, “A Functionalizable Polyester with Free Hydroxyl Groups and Tunable Physiochemical and Biological Properties.” Biomaterials, 31 3129–3138. https://doi.org/10.1016/j.biomaterials.2010.01.023 (2010)
Chen, Y, Tan, L, Chen, L, Yang, Y, Wang, X, “Study on Biodegradable Aromatic/Aliphatic Copolyesters.” Braz. J. Chem. Eng., 25 321–335. https://doi.org/10.1590/S0104-66322008000200011 (2008)
Tu, Y-M, Wang, X-M, Yang, X, Fan, H-Z, Gong, F-L, Cai, Z, Zhu, J-B, “Biobased High-Performance Aromatic-Aliphatic Polyesters with Complete Recyclability.” J. Am. Chem. Soc., 143 20591–20597. https://doi.org/10.1021/jacs.1c10162 (2021)
Seithümmer, J, Öztürk, M, Wunschik, DS, Prießen, J, Schultz, HJ, Dornbusch, M, Gutmann, JS, Hoffmann-Jacobsen, K, "Enzymatic Synthesis of Novel Aromatic-Aliphatic Polyesters with Increased Hydroxyl Group Density." Biotechnol. J., 17(6)2100452. https://doi.org/10.1002/biot.202100452 (2022)
Brock, T, Groteklaes, M, Mischke, P, European Coatings Handbook, 2nd edn. Vincentz Network, Hannover (2010)
Zhao, F, Bi, W, Zhao, S, “Influence of Crosslink Density on Mechanical Properties of Natural Rubber Vulcanizates.” J. Macromol. Sci. B, 50 1460–1469. https://doi.org/10.1080/00222348.2010.507453 (2011)
Dornbusch, M, Christ, U, Rasing, R, Epoxy Resins: Fundamentals and Applications. Vincentz Network, Hanover (2016)
Page, AJ, Chou, C-P, Pham, BQ, Witek, HA, Irle, S, Morokuma, K, “Quantum Chemical Investigation of Epoxide and Ether Groups in Graphene Oxide and Their Vibrational Spectra.” Phys. Chem. Chem. Phys., 15 3725–3735. https://doi.org/10.1039/c3cp00094j (2013)
Socrates, G, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd edn. Chichester, Wiley (2010)
Hesse, M, Meier, H, Zeeh, B, Spectroscopic Methods in Organic Chemistry: 100 Tables, 2nd edn. Thieme, Stuttgart (2008)
Zhao, C, Huang, C, Chen, Q, Ingram, IDV, Zeng, X, Ren, T, Xie, H, "Sustainable Aromatic Aliphatic Polyesters and Polyurethanes Prepared from Vanillin-Derived Diols via Green Catalysis." Polymers, 12 (3) 586. https://doi.org/10.3390/polym12030586 (2020)
Knospe, P, Böhm, P, Gutmann, J, Dornbusch, M, “Oxazoline-Based Crosslinking Reaction for Coatings.” J. Coat. Technol. Res., 18 1199–1207. https://doi.org/10.1007/s11998-021-00479-9 (2021)
Takeuchi, H, Watanabe, N, Harada, I, “Vibrational Spectra and Normal Coordinate Analysis of p-Cresol and Its Deuterated Analogs.” Spectrochim. Acta A, 44 749–761. https://doi.org/10.1016/0584-8539(88)80138-7 (1988)
Bumbac, M, Zaharescu, T, Nicolescu, CM, “Thermal and Radiation Stability of Alkyd Based Coatings Used as Insulators in the Electrical Rotating Machines.” J. Sci. Arts, 17 119–130 (2017)
Wysoglad, J, Einflüsse der Schichtstrukturen von Coil-Coating-Beschichtungssystemen und deren Eigenschaftsprofile, DuEPublico: Duisburg-Essen Publications online. University of Duisburg-Essen, Germany (2021)
Larkin, PJ, Makowski, MP, Colthup, NB, “The Form of the Normal Modes of s-triazine: Infrared and Raman Spectral Analysis and Ab Initio Force Field Calculations.” Spectrochim. Acta A, 55 1011–1020. https://doi.org/10.1016/S1386-1425(98)00244-3 (1999)
Acknowledgments
This work was supported by the German “Bundesministerium für Bildung und Forschung (BMBF)” in the framework of the program “FHProfUnt”, Grant 13FH125PX6, and Covestro Deutschland AG.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knospe, P., Seithümmer, J., Reichmann, R. et al. Impact of enzymatically synthesized aliphatic–aromatic polyesters with increased hydroxyl group content on coating properties. J Coat Technol Res 19, 1799–1808 (2022). https://doi.org/10.1007/s11998-022-00651-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00651-9