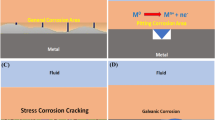
Abstract
Coatings have been used since time immemorial for decorative and protective purposes. During the initial periods, when not much information was available regarding the health impact of the raw materials, there was no thought given or restrictions put on use of any ingredients in coatings. Some of the raw materials like white lead (lead carbonate), red lead (lead oxide), hexavalent chromium compounds, and other similar compounds were being used in large quantities to get specific paint film properties. This not only affected human health but also with no effective effluent treatment contaminated soil and water resources. With progress in technology and diagnostics, lead and chromium compounds have been identified as carcinogens. The developed countries (Europe and USA) were the first to ban the use of lead in their products in the 1970s and 1980s, but due to mainly economic reasons, these are still being used in the developing countries. As the long-term health impact is too much to bear, regulations/legislations have been enacted by the governments restricting use of lead/chrome in paints. The Global Alliance to Eliminate Lead Paint (GAELP), a joint association formed under the United Nations Environment programme, and World Health Organization have agreed to phase out manufacture and sale of paints containing lead by 2020. Similar restrictions exist for many other compounds used in coatings. The article attempts to present a synopsis of the history of coatings composition, impact of the heavy metals on health and environment, and the options available for shifting to safer coating composition. As major focus of the world is on lead elimination, it has been covered in more detail, but the article also provides information on other metals, namely cadmium, chromium, mercury, arsenic, antimony, tin, nickel, manganese, cobalt, etc., and their current status.









Similar content being viewed by others
References
“Lead in Paints.” Dr. Sapna Johnson Dr. Nirmali Saikia Mr. Ramakant Sahu, Centre for Science and Environment, Date August 2009,PML/PR-34/2009, (https://www.researchgate.net/profile/Ramakant_Sahu/publication/238732631_Lead_in_Paints/links/0deec529c276591ab8000000.pdf), accessed on 17 April 2020
https://en.wikipedia.org/wiki/Lead, accessed on 06 May 2020
“Global Lead Paint Elimination by 2020: A Test of the Effectiveness of the Strategic Approach to International Chemicals Management.” Jack Weinberg IPEN Senior Policy Advisor, Dr. Scott Clark IPEN Public Health Advisor for Lead Paint Professor Emeritus, Environmental Health University of Cincinnati, (https://ipen.org/pdfs/ipen_global_lead_paint_elimination_report_2012.pdf), accessed on 17 April 2020
“Development and Preparation of Lead-Containing Paint Films and Diagnostic Test Materials.” David Binstock, William Gutknecht, Kristen Sorrell, Curtis Haas, Wayne Winstead, Michelle McCombs, Gordon Brown, and Cynthia Salmons, and Sharon L. Harper
https://www.britannica.com/science/lead-chemical-element, accessed on 06 May 2020
“Toolkit for Establishing Laws to Control the Use of Lead in Paint.” Global Alliance to Eliminate Lead Paint.
OCCA Student Monograph No. 1 Basic Science for Students of Paint Technology, 1998, p. 19
https://en.wikipedia.org/wiki/Environmental_impact_of_paint “Environmental Impact of Paint.” From Wikipedia, the Free Encyclopaedia Lit 4
Tapan K. Debroy, Utica; Ding Y.Chung, Rochester Hills; Craig R.Deschner, Southfield; Sioe-Heng A.Tjoe, Troy, all of Mich. Feb. 20, 1990, “Cathodic Electrodeposition Coatings Containing Lead Cyanamideas A Supplementary Catalyst.” Patent Number: 4,994
“Model Law and Guidance for Regulating Lead Paint.” The United Nations Environment Programme Revised July 2018, UN Environment, Global Alliance To Eliminate Lead Paint, (https://wedocs.unep.org/bitstream/handle/20.500.11822/22417/Model_Law_Guidance_%20Lead_Paint.pdf?sequence=7), accessed on 27 April 2020
Study on Lead in Paints in India 2019, IPEN a Toxic Free Future, Supervised by: Piyush Mohapatra, Research and Compiled by: Tripti Arora, Dr. Prashant Rajankar, (http://toxicslink.org/docs/Lead%20in%20Paints%202%20(4).pdf), accessed on 27 April 2020
“LEAD: Time To Retire From Continuous Galvanizing and Organic Coating in Flat Steel Industry.” 19th Middle East Iron & Steel Conference. 14 – 16th Dec 2015
“Lead in New Decorative Paints: A Global Study.” By Dr Abhay Kumar, IPEN, August 2009, Toxics Link, H2 Jungpura Extension, New Delhi-110014. www.toxicslink.org email: info@toxicslink.org, (http://pan-afrique.org/fr/Rapports/Etudes/Global_Lead_in_Paint.pdf), accessed on 27 April 2020
“Lead-free Paints.” Source: Insight-The Consumer Magazine September-October 2009,http://cercenvis.nic.in/PDF/eco%20friendly%20paint.pdf, accessed on 18 April 2020
“Current Status and Future of Lead Based Paints and Pigments in Asia and the Pacific-Interim Report.” Satoshi Murao and Kyoko Ono, September 2012, TsukubaJapan, (https://wedocs.unep.org/bitstream/handle/20.500.11822/14241/Interim_UNEP_AIST-as%20of%2018Sep-final clean%20%281%29.pdf?sequence=1&isAllowed=y)), accessed on 16 April 2020
“Global Report on the Status of Legal Limits of Lead in Paints-2016 Report.” United Nations Environment Programme | May 2016, (https://wedocs.unep.org/bitstream/handle/20.500.11822/11348/Limits-Lead-Paint-2016%20Report-Final.pdf?sequence=1&isAllowed=y), accessed on 05 May 2020
https://www.chemistryworld.com/newfs/why-use-lead-in-paint/3004319.article “Why Use Lead in Paint?” By James Mitchell Crow 21 August 2007
“Technical Guidelines for Replacing Lead Oxide in Anti-corrosives Paints in Tunisia.” July 2018
“Update on the Global Status of Legal Limits on Lead in Paint.” September 2019, (https://wedocs.unep.org/bitstream/handle/20.500.11822/30110/2019_Global_Update.pdf?sequence=1&isAllowed=y), accessed on 03 May 2020
“Replacement of Lead Pigments in Solvent Based Decorative Paints.” IPEN- A Toxic Free Future, (https://ipen.org/sites/default/files/documents/Replacement%20of%20lead%20pigments%20in%20solvent%20based%20decorative%20paints.pdf), accessed on 10 May 2020
“Alternatives for Lead Chromate Based Pigments in the Coatings Industry and Their Technical Compromise.” Cristina Zanzottera, PhD, MBA Product Manager Dominion Colour Corporation Maastricht, The Netherlands, (http://www.mypma.org.my/downloads/events/green-1117/5-lead-free-alternatives.pdf), accessed on 30 March 2020
“Lead Free for Our Environment.” Heubach, (https://www.heubach-india.com/fileadmin/downloads/brochures/Bleifrei_web_1.pdf), accessed on 30 March 2020
EN ISO 18451-1 Pigment Models
“Wetting and Dispersing Additives.” BYK Additives and Instruments, (https://ebooks.byk.com/fileadmin/pdf/BYK_L-WI_1_EN_Online.pdf), accessed on 10 May 2020
“Little Helpers Love Great Achievements Practical Guide to Dispersing Agents.”- BASF, accessed on 30 October 2021
“Hybrid Pigments.” European Coatings Journal, 06/2008, Vincentz
“Pigmentation Alternates to Lead Chromates.” UmitHancer, October 2019 by BASF, (http://www.saicm.org/portals/12/Documents/GEF-Project/Beijing-WS/BCE-Leadfree-Alternatives.pdf), accessed on 05 May 2020
“Lead Replacement in Industrial Paints MC Industrial.” January- 2002 Ciba
(https://www.pcimag.com/articles/101063-lead-chromate-replacement) “Lead Chromate Replacement - Old Hat But Still a Long Process.”
(https://www.paint.org/coatingstech-magazine/articles/greater-demand-performance-functionality-drive-pigment-development/) “Greater Demand for Performance and Functionality Drive Pigment Development” Lit 6
“Organic Pigments for Decorative and Industrial Coatings.” (https://www.unioncolours.com/media/1074/union-colours-coatings-brochure-issue-3.pdf), accessed on 25 April 2020
Malshe, VC, Basics of Paint Technology. 2002, pp. 78-79
Corrosion and Corrosion Control, an Introduction to Corrosion Science and Engineering. Fourth Edition R. Winston & Herbert H. Uhlig 2008 by John Wiley & Sons, Inc. p. 291
“Lead Paint Reformulation Technical Guidelines.” (http://www.saicm.org/Portals/12/Documents/GEF-Project/Lead-Paint/Lead_Paint_TG_Draft_25032019.pdf), accessed on 30 April 2020
The Chemistry and Technology of Paints. Maximilian Toch, 1916, p. 177
https://www.nature.com/articles/s41529-018-0034-5 “Chromate Replacement: What Does the Future Hold?”
NR Whitehouse, PRA, MeltonMowbray,Leicestershire, UK, Management of Corrosion of Automobiles. 2017 Elsevier Inc.
Pipe Line Corrosion & Cathodic Protection. Third Edition, Marshall E. Parker Edward G. Peattie, 1999, by Elsevier Science (USA), p. 126
https://www.gravitaindia.com/litharge/, accessed on 17 May 2020
https://en.wikipedia.org/wiki/Cadmium, accessed on 14 June 2020
Cadmium Review, Nordic Council of Ministers, January 2003
https://www.cadmium.org/cadmium-applications, accessed on 14 June 2020
https://en.wikipedia.org/wiki/Cadmium_pigments, accessed on 14 June 2020
“Exposure to Cadmium: A Major Public Health Concern, Preventing Disease Through Healthy Environments.” World Health Organisation
“Beyond Cadmium To More Colours!” The Art Tree House, September 23, 2018 (https://arttreehouse.com/artstore/beyond-cadmium-to-more-colors/)
“Cadmium in General and Copper-based Paints.” European Chemicals Agency (ECHA), 19 November 2012
Brandes, EA, Greenaway, HT, Stone, HEN, “Ductility in Chromium.” Nature 178 (4533) 587 (1956)
EPA (August 2000). “Abandoned Mine Site Characterization and Clean up Handbook.” (PDF). United States Environmental Protection Agency. Retrieved 8 September 2019
Morrison, RD; Murphy, BL (4 August 2010). Environmental Forensics: Contaminant Specific Guide. Academic Press. ISBN 9780080494784
Marrion, Alastair (2004). The Chemistry and Physics of Coatings. Royal Society of Chemistry. pp. 287. ISBN 978-0-85404-604-1
(https://colourlex.com/project/viridian), accessed on 08 July 20
“Sampling and Monitoring for Lead.” https://www.cpwr.com/sites/default/files/training/lead/Lead%209.pdf), accessed on 05 May 2020
Senese, F. “Why is Mercury a Liquid at STP?” General Chemistry Online at Frostburg State University. Archived from the Original on 4 April 2007, accessed on 16 June 2020
Merck's Manual 1899 (First ed.). Archived from the Original on 24 August 2013, accessed on 16 June 2020
Kulshreshtha, AK, Basic Electrical Engineering: Principles and Applications. Tata McGraw-Hill Education, India (2009)
“https://www.cdc.gov/mmwr/preview/mmwrhtml/00001566.htm”, accessed on 16 June 2020
King, RJ, “Minerals Explained 37: Cinnabar.” Geology Today. https://doi.org/10.1046/j.0266-6979.2003.00366.x (2002)
“Mercury.” NIEHS, Archived from the Original on 19 November 2016, accessed on 16 June 2020
Bose-O’Reilly, S, McCarty, KM, Steckling, N, Lettmeier, B, “Mercury Exposure and Children’s Health.” Curr. Probl. Pediatr. Adolesc. Health Care. https://doi.org/10.1016/j.cppeds.2010.07.002 (2010)
Rooney, JPK, “The Retention Time of Inorganic Mercury in the Brain — A Systematic Review of the Evidence.” Toxicol. Appl. Pharmacol.,. https://doi.org/10.1016/j.taap.2013.12.011 (2014)
https://en.wikipedia.org/wiki/Arsenic, accessed on 17 June 2020
“Arsenic and Arsenic Compounds.” (https://www.ncbi.nlm.nih.gov/books/NBK304380/), accessed on 17 June 2020
“Arsenic and Old Paint.” New Scientist, 29 May 1999, https://www.newscientist.com/article/mg16221883-400-arsenic-and-old-paint/, accessed on 17 June 2020
“Non-silicone Biocide-free Antifouling Solutions.” J A Lewis, ES Link Services Pty Ltd, Australia
“The Arsenic Rule: Background and Rule Provisions.” https://www.epa.gov/sites/production/files/2015-09/documents/train1-background.pdf, accessed on 17 June 2020
Meija, J, et al. “Atomic Weights of the Elements 2013 (IUPAC Technical Report).” Pure Appl. Chem. https://doi.org/10.1515/pac-2015-0305 (2016)
“www.http://pubs.usgs.gov/of/2003/of03-019/of03-019.pdf”, Butterman, C.; Carlin, Jr., J. F. (2003). “Mineral Commodity Profiles: Antimony.” United States Geological Survey, accessed on 18 June 2020
O'Mara, William C.; Herring, Robert B.; Hunt, Lee Philip (1990). Handbook of Semiconductor Silicon Technology. William Andrew. p. 473. ISBN 978-0-8155-1237-0. Fastener. In Glass and Ceramics, Sb is Present as a Pigment, Fining Agent, Opacifier or Fastener.
Weil, Edward D.; Levchik, Sergei V. “Antimony Trioxide and Related Compounds.” Flame Retardants for Plastics and Textiles: Practical Applications.
“https://www.atsdr.cdc.gov/toxprofiles/tp23.pdf”, “Toxicological Profile for Antimony and Compounds.” October 2019, accessed on 19 June 2020
“www.https://www.britannica.com/science/antimony-poisoning”, “Antimony Poisoning.” Encyclopaedia Britannica, accessed on 19 June 2020
Sundar, S, Chakravarty, J, “Antimony Toxicity.” Int. J. Environ. Res. Public Health, 7 (12) 4267–4277. https://doi.org/10.3390/ijerph7124267 (2010)
https://en.wikipedia.org/wiki/Tin accessed on 10 June 2020
https://www.lenntech.com/periodic/elements/sn.htm accessed on 10 June 2020
“Tributyltin.” Toxicpedia, Katarina Lah, Last Updated by Toxipedia in 2011, https://www.healthandenvironment.org/docs/ToxipediaTributylTinArchive.pdf, accessed on 10 June 2020
Lagerstrom, M, Strand, J, Eklund, B, Ytreberg, E, “Total Tin and Organotin Speciation in Historic Layers of Antifouling Paint on Leisure Boat Hulls.” Environ. Pollut., 220 1333–1341 (2017)
Dieter Guhl, TIB Chemicals AG, Mannheim, Germany, “Alternatives to DBTL Catalysts in Polyurethanes – A Comparative Study.” Research Gate, Access on 12 March 2020
https://www.esaar.com/2018/07/08/cuprous-oxide-red-what-is-it-how-is-it-used/without-antifouling-paint-coating/, accessed on 14 June 2020
Voulvoulis, N, Scrimshaw, MD, Lester, JN, “Review-Alternative Antifouling Biocides.” Appl. Organometal. Chem., 13 135–143 (1999)
Anti-fouling Systems, Focus on IMO, International Maritime Organization, 4 Albert Embankment, London SE1 7SR, United Kingdom
“Redefining Antifouling Coatings.” Dr. Geoffrey Swain, Oceanography and Ocean Engineering, Florida Institute of Technology, Melbourne, FL, U.S.
SIKORA, Robert, John Northville, MI 48167 (US), Date of filing: 02.12.1999, “Bismuth Oxide Catalyst for Cathodic Electrocoating Compositions.” EP 1 135 443 B1
“Nickel Use in Society.” Nickel Institute, Archived from the Original on September 21, 2017, accessed on 07 July 20
Davis, Joseph R. (2000), “Uses of Nickel.” ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys. ASM International, pp. 7–13, ISBN 978-0-87170-685-0
https://www.heubachcolor.com/products/inorganicpigments/#:~:text=Nickel%20rutiles%20are%20light%20lemon,shade%20of%20the%20final%20product, accessed on 07 July 20
Ololade, IA, Oginni, O, “Toxic Stress and Hematological Effect on African Catfish Clarias gariepinus, Fingerlings.” J. Environ. Chem., 2 (2) 014–019 (2010)
Butticè, Claudio (2015), “Nickel Compounds,” In Colditz, Graham A. (ed.), The SAGE Encyclopedia of Cancer and Society (Second ed.), Thousand Oaks: SAGE Publications, Inc. pp. 828–831, ISBN 9781483345734
Ewa BJ, Mikomaj P, “Effects of Cadmium and Nickel on Hematological Parameters of Common Carp, Cyprinus carpio L.”, Acta Ichthyologica Et Piscatoria. (2005)
Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). “Mangan.” Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1110–1117. ISBN 978-3-11-007511-3
http://www.americanmanganeseinc.com/wp-content/uploads/2011/08/American-Manganese-Phase-II-August-19-2010-Final-Report-Internet-Version-V2.pdf, accessed on 08 July 20
https://www.suppliersonline.com/propertyp.s/2024.asp, accessed on 08 July 20
https://www.govinfo.gov/app/details/FR-1995-07-17/95-17476, accessed on 08 July 20
Rehsi, S.S. (31 December 1983), Magnesium Oxide in Portland Cement, pp. 467–483, ISBN 9780080286709. accessed on 08 July 20
Basics of Paint Technology, by VC Malshe, 2002, p. 46.
http://cameo.mfa.org/wiki/Manganese_dioxide#:~:text=Both%20natural%20and%20synthetic%20manganese,as%20a%20colorant%20in%20mortar.&text=It%20is%20used%20as%20a,a%20drier%20for%20oil%20paints, accessed on 08 July 20
https://www.handprint.com/HP/WCL/pigmt1b.html, accessed on 08 July 20
Erikson, Keith M.; Ascher, Michael (2019). “Chapter 10. Manganese: Its Role in Disease and Health.” In Sigel, Astrid; Freisinger, Eva; Sigel, Roland K. O.; Carver, Peggy L. (Guest editor) (eds.). Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic. Metal Ions in Life Sciences. 19. Berlin: de Gruyter GmbH. pp. 253–266.
Emsley, John (2001). “Manganese.” Nature’s Building Blocks: An A-Z Guide to the Elements. Oxford, UK: Oxford University Press. pp. 249–253. ISBN 978-0-19-850340-8.
Murthy, V. S. R (2003), “Magnetic Properties of Materials.” Structure And Properties Of Engineering Materials, p. 381, ISBN 978-0-07-048287-6
Holleman, AF, Wiberg, E, Wiberg, N, (2007), “Cobalt.” Lehrbuch der Anorganischen Chemie (in German) (102nd ed.), de Gruyter, pp. 1146–1152, ISBN 978-3-11-017770-1
http://www.britannica.com/EBchecked/topic/123235/cobalt-Co, accessed on 08 July 20
Campbell, Flake C (2008-06-30), “Cobalt and Cobalt Alloys.” Elements of Metallurgy and Engineering Alloys, pp. 557–558, ISBN 978-0-87170-867-0
Michel, R, Nolte, M, Reich, M, Löer, F, “Systemic Effects of Implanted Prostheses Made of Cobalt-Chromium Alloys.” Arch. Orthoped. Trauma Surg., 110 (2) 61–74. https://doi.org/10.1007/BF00393876 (1991)
Overman, Frederick (1852). A Treatise on Metallurgy. D. Appleton & Company, pp. 631–637
Muhlethaler, B, Thissen, J, Muhlethaler, B, “Smalt.” Stud. Cons., 14 (2) 47–61. https://doi.org/10.2307/1505347 (1969)
Committee on Technological Alternatives for Cobalt Conservation, National Research Council (U.S.); National Materials Advisory Board (1983), “Ground–Coat Frit,” Cobalt Conservation Through Technological Alternatives. p. 129
Handbook of Coatings Additives. Edited by Leonard J. Calbo. King Industries, Norwalk, Connecticut, p. 492.
Donaldson, John D. and Beyersmann, Detmar (2005) “Cobalt and Cobalt Compounds.” Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi: https://doi.org/10.1002/14356007.a07_281.pub2
NIOSH Pocket Guide to Chemical Hazards. “#0146.” National Institute for Occupational Safety and Health (NIOSH)
Elbagir, Nima; van Heerden, Dominique; Mackintosh, Eliza (May 2018), “Dirty Energy.” CNN, Accessed on 07 July 20
Basketter, DA, Angelini, G, Ingber, A, Kern, PS, Menné, T, “Nickel, Chromium and Cobalt in Consumer Products: Revisiting Safe Levels in the New Millennium.” Contact Dermatitis, 49 (1) 1–7. https://doi.org/10.1111/j.0105-1873.2003.00149.x (2003)
Charlotte Pilemand and Eva Wallström EnPro ApS, “Substitution of Cobalt Driers and Methyl Ethyl Ketoxime.” accessed on 20 March 2020
https://www.lenntech.com/periodic/elements/zr.htm#:~:text=Zirconium%20is%20a%20very%20strong,hardness%20is%20similar%20to%20copper accessed on 14 December 2020
“Pigment & Resin Technology.” Vol. 7 Issue 9 pp. 15–17, (https://doi.org/10.1108/eb041421)
https://www.pfonline.com/articles/conversion-coatings-phosphate-vs-zirconium-,accessed on 21 March 2022
https://en.wikipedia.org/wiki/Zirconium#Safety, accessed on 07 July 2020
https://www.cdc.gov/niosh/npg/npgd0677.html, accessed on 07 July 2020
https://en.wikipedia.org/wiki/Titanium#:~:text=Titanium%20is%20a%20chemical%20element,%2C%20aqua%20regia%2C%20and%20chlorine accessed on 14 December 2020
https://www.chemicalsafetyfacts.org/titanium-dioxide/, accessed on 07 July 2020
“Pigments Through the Ages - History - Titanium Dioxide Whites.” (webexhibits.org), accessed on 20 March 2022
https://echa.europa.eu/brief-profile/-/briefprofile/100.013.894 accessed on 20 March 2022
“Brands and Products.” | The Chemours Company accessed on 20 March 2022
GS-11 Paints and Coatings 2nd Ed (greenseal.org) p. 19 accessed on 20 March 2022
“Hazardous Substance Fact Sheet, Titanium Dioxide, New Jersey Department of Health.” https://nj.gov/health/eoh/rtkweb/documents/fs/1861.pdf, accessed on 07 July 2020
https://en.wikipedia.org/wiki/Zinc#:~:text=Zinc%20is%20a%20chemical%20element,appearance%20when%20oxidation%20is%20removed.&text=Zinc%20is%20the%2024th%20most,and%20has%20five%20stable%20isotopes accessed on 14 December 2020
https://www.lenntech.com/periodic/elements/zn.htm, accessed on 07 July 2020
“The Essential Toxin: Impact of Zinc on Human Health.” Laura M. Plum, Lothar Rink, and Hajo Haase, Int J Environ Res Public Health. 2010 Apr; 7(4): 1342–1365, Published online 2010 Mar 26. doi: https://doi.org/10.3390/ijerph7041342
“Zinc Metal Safety Data Sheet.” Teck,https://www.teck.com/media/Zinc-Metal-SDS.pdf, accessed on 07 July 2020.
“Brief Guide to Analytical Methods for Measuring Lead in Paint, Inter-Organization Programme for the Sound Management of Chemicals.” World Health Organization 2011, https://www.who.int/ipcs/assessment/public_health/lead_paint.pdf), accessed on 01 May 2020
“Lead Requirements for Contractors Conducting Work at The University of Texas at Austin.” https://ehs.utexas.edu/programs/occupationalsafety/documents/Lead-Requirements-Contractors.pdf, accessed on 03 May 2020
“Analytical Methods for Measuring Lead in Paints.” http://www.saicm.org/Portals/12/Documents/GEF-Project/Amalty-WS/Analysis%20of%20lead%20in%20paints_ENG.pdf accessed on 03 May 2020
http://chemicalinstrumentation.weebly.com/flame-aas.html, accessed on 03 May 2020
https://www.astm.org/Standards/D564.htm, accessed on 07 July 20
https://www.astm.org/Standards/D2350.htm, accessed on 17 June 2020
https://www.astm.org/Standards/D2373.htm, accessed on 07 July 20
https://www.astm.org/DATABASE.CART/HISTORICAL/D2375-05.htm, accessed on 07 July 20
(https://www.astm.org/Standards/D3335.htm, accessed on 07 July 20
https://www.astm.org/Standards/D3624.htm, accessed on 16 June 2020
https://www.astm.org/Standards/D3717.htm, accessed on 17 June 2020
https://www.osha.gov/dts/sltc/methods/inorganic/id103/id103.html, accessed on 16 June 2020
Acknowledgment
The authors wish to thank Asian Paints management for giving the opportunity to publish the review paper. The authors also wish to thank Dr Rajeev Jain, Ms Elvina Rose, Dr Shekhar Tambe, Dr Ramashanker Mishra, and Dr Sujay Mahajan for critically reviewing the paper and providing valuable inputs regarding its content.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Puthran, D., Patil, D. Usage of heavy metal-free compounds in surface coatings. J Coat Technol Res 20, 87–112 (2023). https://doi.org/10.1007/s11998-022-00648-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00648-4