Abstract
High-pressure processing (HPP) is a non-thermal preservation technology that can be applied as a control measure to inactivate pathogens and spoilage microorganisms once RTE meat products are packaged in a convenient format. HPP efficacy highly depends on product characteristics, but the impact of the sodium-reduced formulations and the effect of packaging atmosphere are scarcely known. The aim of the present work was to assess the effect of standard and sodium-reduced formulations from two different brands (A, B) under different packaging (vacuum and modified atmosphere packaging (MAP)) on the HPP inactivation kinetics of Listeria monocytogenes and spoilage lactic acid bacteria in cooked ham. Slices of cooked ham with standard and sodium-reduced formulations were inoculated with L. monocytogenes CTC1034 and Latilactobacillus sakei CTC746 (slime producer), packaged in vacuum and MAP (CO2:N2, 20:80), and pressurized (400 MPa/0–15 min) after 1 h (vacuum, MAP) or 24 h (MAP-exposed). Parameters of HPP inactivation kinetics were estimated by fitting the Weibull model to log reduction data. Results showed that the efficacy of HPP in sodium-reduced cooked hams tended to decrease compared to standard formulations, being the difference statistically significant for L. sakei. For L. monocytogenes, a significant enhancing effect of MAP was observed when HPP was applied just after packaging (1 h, MAP) of cooked ham of brand A. In the case of L. sakei, the inactivation by HPP was only enhanced in MAP-exposed samples. Therefore, the use of HPP as a control measure must be applied through a product-oriented approach considering the type of packaging and the time period between packaging and HPP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Listeria monocytogenes is a foodborne pathogen of great concern and the causative agent of listeriosis, a severe illness with the highest case fatalities among foodborne zoonotic diseases (EFSA and ECDC, 2021). This pathogen is also one of the main reasons food alerts and product recalls (Capaldo, 2020; FSANZ, 2023). According to the risk assessments developed by several organizations worldwide, the consumption of ready-to-eat (RTE) cooked meat products commercialized in a convenient format (sliced and packaged, thus exposed to contamination after cooking) ranks among the highest risk of listeriosis (FDA/USDA, 2013; EFSA BIOHAZ Panel, 2018). Food business operators (FBO) seek for strategies to eliminate or reduce L. monocytogenes to increase the safety of their products. In addition, due to market demands and the increased awareness towards food waste, FBO are considering the extension of the shelf life a critical challenge to be addressed through the reduction of the level of spoilage microorganisms, such as lactic acid bacteria (LAB), that limit the shelf life of pre-packaged cooked meat products from the sensory perspective (Iulietto et al., 2015). In this context, to both increase the safety and extend the shelf life of meat products minimizing the impact on quality characteristics, preservation technologies are applied as kill step or post-lethality treatments, including the use of high-pressure processing (HPP). HPP is a non-thermal technology with a widespread industrial implementation, whose efficacy and costs depend on technological parameters (pressure, holding time, and pressurization fluid (water) temperature), the target microorganism, and the food matrix (EFSA BIOHAZ Panel, 2022). Regarding the impact of the food matrix characteristics, a protective effect of high fat content (Hereu et al., 2012), high NaCl content (Balamurugan et al., 2016), and low aw (Bover-Cid et al., 2015) on the L. monocytogenes HPP inactivation has been reported in meat products.
The inclusion of sodium chloride (NaCl, salt) in the formulation of cooked meat products makes them an important dietary source of sodium (Na) (Desmond, 2006), which is linked with hypertension and the consequent increased risk of coronary heart diseases and mortality (Messerli et al., 2021). Initiatives of public health authorities forcing meat industry to lower the salt concentration were lunched more than one decade ago (WHO, 2004; MHCA, 2005) and have been taken by food business operators as an opportunity to reformulate meat products and to add nutritional claims appreciated by consumers. Scarce information is available about the impact of sodium-reduced formulations on the efficacy of HPP. Available studies have dealt with the individual impact of different concentrations of NaCl in meat products/systems on HPP lethality showing lower inactivation at increasing amounts of NaCl (Balamurugan et al., 2016; Teixeira et al., 2016). Nevertheless, the development of sodium-reduced meat products is not straightforward, and the replacement of the technological and organoleptic role of NaCl is a challenge (Ruusunen & Puolanne, 2005; Ruusunen et al., 2001). Therefore, the development of sodium-reduced meat products often involves the replacement of NaCl by other salts, especially potassium chloride, and the addition of sugars (Desmond, 2006; Teixeira et al., 2021; Weiss et al., 2010), among others, which may also influence the efficacy of HPP. Moreover, some producers formulate cooked meat products with additives such as salts of organic acids, i.e., lactate, as a humectant that compensate the reduction of NaCl. Lactate also shows antimicrobial activity reducing the growth L. monocytogenes and LAB, thus constituting a natural preservative (Devlieghere et al., 2009). However, lactate also exerts a significant piezoprotection on L. monocytogenes, thus reducing the efficacy of HPP (Serra-Castelló et al., 2021a). Packaging strategies such as vacuum (VP) or modified atmosphere packaging (MAP, with CO2) are also applied to reduce the growth rate of L. monocytogenes and/or lactic acid bacteria aiming to enhance safety and to extend the shelf life by reducing the growth of both pathogens and spoilage bacteria (Devlieghere et al., 1999; Szalai et al., 2004).
To the authors’ knowledge, there are no studies dealing with the impact of packaging of cooked meat products with conventional and sodium-reduced formulations containing (or not) antimicrobial preservatives on the efficacy of HPP when applied as an intervention strategy for reducing the levels of L. monocytogenes and spoilage lactic acid bacteria. Accordingly, the aim of the present study was to evaluate the inactivation behavior L. monocytogenes and slime-producing Latilactobacillus sakei against HPP applied to cooked ham with standard and sodium-reduced cooked ham formulations, with and without lactate, under different convenient packaging systems (vacuum, MAP).
Material and Methods
Cooked Ham Description and Characterization
Cooked hams belonging to two different commercial brands and producers were used in the present study (Table 1), including products formulated with natural preservatives, e.g., lactate (brand A), and products formulated without lactate (brand B). From each brand, one product with standard formulation and one with the claim of sodium-reduced formulation were used. For each type, two whole pieces of cooked ham were purchased directly from the producers and kept under refrigeration (2 ± 1 °C) until used.
AquaLab™ instrument (Series 3; Decagon Devices Inc., Pullman, WA, USA) was used to measure product aw. The pH was measured with a penetration prove (52–32; Crison Instruments SA, Alella, Spain) connected to a portable pH meter (PH 25; Crison Instruments) and calibrated with three buffer solutions of pH 4.01, 7.00, and 9.21 (Crison Instruments) at the sample temperature. Flame atomic absorption spectroscopy was used to determine sodium content (Anonymous, 1979). Total lactate/lactic acid (D + L) was determined using commercial kit D/L-Lactic Acid (D-/L-Lactate) Assay kit from Megazyme International (Wicklow, Ireland) following manufacturer instructions. Concentrations of CO2, N2, and O2 inside packages were measured with the gas analyzer PBI Dansensor CheckMate II (AMETEK Instrumentos, S.L.U., Barcelona). Analysis was performed in triplicate.
Preparation of L. monocytogenes and L. sakei Cultures
The L. monocytogenes CTC1034 strain (serotype 4b) isolated from a meat product and previously used in HPP studies (Bover-Cid et al., 2019; Hereu et al., 2012; Serra-Castelló et al., 2021a) and the slime producer L. sakei CTC746 strain isolated from ropy slime sliced cooked ham (Aymerich et al., 2002; Garriga et al., 1998) were used in the present study, both strains belonging to the IRTA-Food Safety and Functionality Program collection. A loopful of frozen stock culture (− 80 °C) of L. monocytogenes CTC1034 strain was grown at 37 °C on Plate Count Agar (PCA, Merck, Darmstadt, Germany) for 18 h. Then, one colony was picked and seeded in PCA to reach confluent growth 37 °C overnight. For L. sakei CTC746, a loopful of the frozen stock culture (− 80 °C) was grown at 30 °C on MRS agar (de Man, Rogosa, and Sharpe, Merck, Darmstadt, Germany) for 72 h under anaerobiosis using sealed jars with an AnaeroGen sachet (Oxoid Ltd.). To reach confluency, one colony of L. sakei was picked, seeded, and grown in MRS agar for 72 h under anaerobiosis. To collect and resuspend the bacterial biomass of both strains, a cryoprotectant solution consisting of 0.3% of beef extract (Difco Laboratories, Detroit, MI, USA), 0.5% of tryptone (Oxoid Ltd., Basingstoke, Hampshire, UK), and 20% of glycerol was used (Serra-Castelló et al., 2022). Cultures were stored at – 80 °C until use.
Inoculation, Packaging, and HPP
Cultures of L. monocytogenes CTC1034 and L. sakei CTC746 were thawed at room temperature and inoculated at 0.5% v/w onto 1.5 mm slices of cooked ham to achieve 108–109 cfu/g, spreading it with a sterile spreader until absorbed. Individual samples consisting of one inoculated slice overlaid with one non-inoculated slice were prepared and packaged (EV-15–2-CD vacuum packer; Tecnotrip, Terrassa, Spain) in PA/PE plastic bags (oxygen permeability of 50 cm3/m2/24 h and a low water vapor permeability of 2.8 g/m2/24 h; Sistemvac, Estudi Graf S.A., Girona, Spain). Three treatments were applied including two different packaging systems: packaging samples in (i) vacuum (VP) and (ii) modified atmosphere (20% CO2:80% N2) 1 h prior to pressurization (MAP) and (iii) in modified atmosphere (20% CO2:80% N2) 24 h before pressurization (MAP-exposed samples) keeping them under refrigeration (4 ± 1 °C) until HPP.
Cooked ham samples were pressurized at 400 MPa, and holding times of 0 (i.e., a pulse of pressure come-up followed by immediate release), 1.5, 3, 4.5, 7, 10, and 15 min were applied with a Wave 6000 Hiperbaric equipment (Burgos, Spain). The come-up time was 2.5 min, and the pressure release time was almost immediate (< 2 s). The initial temperature of the pressurization fluid (water) was 10 °C. The expected increase in the temperature of the fluid during the treatment was 3 °C for 100 MPa, reaching a total of 22 °C and suggesting no thermal effects on the food matrix (Patazca et al., 2007). Cycles of HPP at predefined conditions of pressure and time were done in different days. For each treatment, three replicates were performed. This resulted in the analysis of a total of 36 non-pressurized cooked ham packages (3 replicates × 3 packaging systems × 4 types of cooked ham) and 252 pressurized cooked ham packages (3 replicates × 7 HPP holding times × 3 packaging systems × 4 types of cooked ham). After pressurization, samples were kept for 1 h at refrigeration temperature before microbiological analysis in order to standardize the procedure among all the assays performed.
Microbiological Determinations
Cooked ham samples (10–15 g) were aseptically cut into small pieces, tenfold diluted in saline solution (0.85% NaCl and 0.1% Bacto Peptone (Becton Dickinson)) in a bag Blender Smasher® (bioMérieux, Marcy-l’Etoile, France) and homogenized for 1 min. Samples were kept at room temperature for 1 h before performing the appropriate tenfold serial dilutions in saline solution. Enumeration of L. monocytogenes CTC1034 was performed on chromogenic agar (CHROMagar™ Listeria; Scharlab, S.L., Sentmenat, Spain) after incubation at 37 °C for at least 48 h to allow injured cells to recover and form colonies. Enumeration of L. sakei CTC746 was performed on MRS agar incubated at 30 °C for at least 72 h under anaerobiosis. Analysis for each set of conditions and microorganism was performed (i.e., 576 microbiological determinations). Petri dishes of 90 and 140 mm diameter were used. The limit of detection was 1 cfu/g by spreading 10 ml into one large Petri dish according to Hunt et al. (2017). The analysis of cooked ham before the challenge tests confirmed that endogenous lactic acid bacterium counts were below the limit of detection.
Primary Inactivation Model Fitting and Statistical Analysis
Counts of L. monocytogenes CTC1034 and L. sakei CTC746 were log transformed, and the inactivation, as log reduction (log N/N0), was calculated using the initial inoculation concentration as reference. The Weibull model (Eq. 1) was fitted to the inactivation data of each microorganism along the HPP holding time to estimate the primary kinetic inactivation parameters (δ and p) quantifying the bacterial resistance to HPP. Data for the three replicates were pooled to compensate possible data correlations and provide a more realistic idea of the variability, which may be underestimated with single level regression, i.e., three different fits, one for each replicate (van Boekel, 2021).
where \({(\mathrm{log}N/{N}_{0})}_{i}\) is the estimated initial bacterial inactivation during come-up time, at t = 0 (i.e., a cycle of pressure come-up and release without holding time), δ is the holding time (min) required for the first log reduction, p is a dimensionless parameter describing the shape of the inactivation curve (i.e., p < 1 concave, p = 1 linear, and p > 1 convex), and t is the holding time (min) during HPP.
The fit of the Weibull model was performed with the least squares method using the nls2 package included in R software (R Core Team, 2019). The goodness of fit was assessed by means of the residual sum of squares (RSS) and the root mean square error (RMSE).
The F-test (Eq. 2) was used to evaluate the statistical differences of the inactivation kinetics between each cooked ham type and for each packaging system (Zwietering et al., 1990).
where RSSNH and dfNH were the residual sum of squares and the degrees of freedom (number of points minus number of parameters of the model), respectively, of the model with common coefficients for the different data sets (null hypothesis) and RSSAH and dfAH were the residual sum of squares and the degrees of freedom, respectively, of the model with specific parameter coefficients for each compared data sets (alternative hypothesis).
Results and Discussion
HPP Inactivation of L. monocytogenes and L. sakei on Cooked Ham of Standard and Sodium-Reduced Formulations
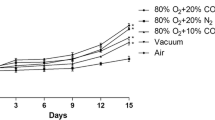
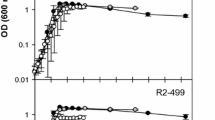
Figure 1 shows the results of HPP inactivation of L. monocytogenes and L. sakei inoculated on cooked ham with standard and sodium-reduced formulations from two different brands (with and without lactate as preservatives), packaged in VP and MAP. The estimated parameters of the inactivation kinetics for each data set are gathered in Table 2. The HPP inactivation kinetics of L. monocytogenes in sodium-reduced products was not statistically different from that observed in their counterpart standard formulation (Fig. 1 and Table 2). In the case of L. sakei, different results were observed depending on the producer and the packaging conditions (Fig. 1). In products of brand B, a significantly lower HPP inactivation was observed in sodium-reduced cooked hams compared with standard formulations packed in both VP and MAP, extending the time for the first log reduction (δ) by 3.4 and 1.4 min, respectively (Table 2). In products of brand A, this was observed only in products pressurized 24 h after packaging in MAP (i.e., MAP-exposed), the δ value in sodium-reduced product being 5 min longer than in the standard product (Table 2). Overall, results showed that sodium-reduced formulations can reduce the efficacy of HPP. As a consequence, the potential higher number of surviving cells after the treatment could achieve the maximum spoilage level earlier, resulting in a shorter shelf life leading to a potentially increased food waste compared to standard formulations. The results obtained in this study differed from those reported in studies dealing with the impact of NaCl increase, where a lower microbial inactivation by HPP due to the higher NaCl content was observed (Balamurugan et al., 2016; Teixeira et al., 2016). However, sodium-reduced cooked hams used in the present study were manufactured by replacing part of NaCl by KCl (Table 1). Similar protective effects of NaCl and KCl had been reported (Balamurugan et al., 2016; Gayán et al., 2013) suggesting that the partial replacement of NaCl by KCl could not relevantly impact on the inactivation of microorganisms by HPP. On the other hand, sorbitol and potato fiber were added in the formulation of sodium-reduced cooked hams of brands A and B, respectively (Table 1), which are known to increase the water holding capacity of meat products (Deis & Kearsley, 2012; Grossi et al., 2012). The presence of these compounds could be partially responsible for the lower inactivation of L. monocytogenes and L. sakei observed in sodium-reduced products since some sugars, including sorbitol, were shown to have protective effect on microbial inactivation by HPP (Setikaite et al., 2009).
Inactivation of L. monocytogenes CTC1034 and L. sakei CTC746 along pressure holding time in cooked hams packaged under VP and MAP just 1 h before HPP and with MAP 24 h before pressurization (MAP-exposed) at 400 MPa. The initial concentration was 108–109 cfu/g. Dotted lines: brand A, continuous lines: brand B, black lines: standard (S) formulation, gray lines: sodium-reduced (SR) formulation
The impact of the different matrix characteristics is complex, and the effect of a specific component may be counteracted by others and/or their interaction. Previous studies have shown an increased microbial resistance against HPP at increasing concentration of salts and/or sugars in laboratory media, model systems, and meat (Balamurugan et al., 2016; Gayán et al., 2013; Koseki & Yamamoto, 2007; Molina-Höppner et al., 2004; Oxen & Knorr, 1993). This can be partially explained by the ability of salts to decrease aw levels in food (Leistner, 2000), since the low aw has been reported to have a piezo-protective effect on microorganisms (Bover-Cid et al., 2015). However, the aw of the cooked hams used in the present study was not different (Table 1) indicating that aw was not the factor responsible for the observed piezo-protective effect of cooked ham formulations. In this sense, previous studies have shown that, besides the aw value, the protection of microorganisms against HPP is dependent on both the nature, i.e., specific ion effect (Hauben et al., 1998; Zhang & Cremer, 2006) and the concentration of the solute, with divalent salts conferring a greater piezo-protective effect compared to monovalent salts (Gayán et al., 2013).
In the present study, the inactivation of both L. monocytogenes and L. sakei was markedly lower in cooked hams with lactate (brand A) compared to cooked hams without lactate (brand B) (Fig. 1). More specifically, δ values were between 0.3 and 4.7 min longer for L. monocytogenes and 6.1 and 11.8 min longer for L. sakei in cooked hams with lactate (brand A) compared to δ values found in cooked hams without lactate (brand B). Lactate has been reported to protect L. monocytogenes and reduce the lethal effects of HPP (Serra-Castelló et al., 2021a). According to the predictions provided by the model of Serra-Castelló et al. (2021a), the higher amount of lactate in cooked hams of brand A (1.1%) compared to brand B (0.6%) (Table 1) would increase by 0.4 min the time required for the first log inactivation of L. monocytogenes CTC1034. Although this prediction is within the observed range of differences in δ values of L. monocytogenes between cooked hams of both brands, the occurrence of wider differences pointed out the potential impact of other intrinsic characteristics of cooked hams in the microbial inactivation by HPP. Moreover, the magnitude of the impact of specific compounds of cooked hams on the efficacy of HPP could be species-dependent since under VP and MAP, the δ of L. sakei was systematically higher than that of L. monocytogenes in cooked hams of brand A, whereas the opposite was observed in cooked hams of brand B (Table 2).
Impact of Packaging System on HPP Inactivation of L. monocytogenes and L. sakei
The HPP inactivation of L. monocytogenes tended to be higher in MAP samples compared to VP (Fig. 1), although it was only statistically significant for L. monocytogenes in cooked hams of brand A, for which δ value for MAP products was 28% shorter than for VP (Table 2). On the contrary, in MAP-exposed samples, non-significant differences of δ values between MAP-exposed and VP were observed regardless of the brand (Table 2).
In MAP, the inhibitory effect of CO2 is related to the dissolved concentration of CO2 into the water phase of foods (Devlieghere et al., 1998). The CO2 absorption capacity of cooked ham depends on the temperature, product composition, and headspace volume, among others (Sivertsvik & Jensen, 2005). In this line, although the refrigeration temperature (4 °C) at which samples were kept prior to pressurization would favor the transfer rate of CO2 from atmosphere to the product (Devlieghere et al., 2001; Sivertsvik & Jensen, 2005), no relevant differences in the gas composition of the headspace were found between MAP-exposed (18% CO2; 81% N2; 1% O2) and non-exposed (17% CO2; 82% N2; 1% O2) samples immediately before HPP. The presence of CO2 can acidify the brain heart infusion (BHI) medium and also induces changes in the primary metabolism, membrane, morphology, and gene expression of L. monocytogenes mainly in response to the acidification of the intracellular medium caused when CO2 diffuses into the cell (Jydegaard-Axelsen et al., 2004, 2005). However, in the present study, no changes in product pH nor in the concentration of L. monocytogenes during the 24 h of exposure to CO2 were observed, indicating that the possible changes in the physiology of L. monocytogenes would not only be related to changes of the physicochemical characteristic of the matrix. On the other hand, Jydegaard-Axelsen et al. (2004) showed that L. monocytogenes LO28 responded to CO2 and acid similarly using the glutamate decarboxylase complex (gad system), which has been related with acid tolerance (Francis et al., 2007). This could be especially relevant since the impact of MAP on L. monocytogenes inactivation by HPP was only significant in cooked hams formulated with lactate (brand A) (Table 2). Specifically, the pre-exposure of L. monocytogenes CTC1034 to lactate for 1 h prior HPP resulted in a shift of the L. monocytogenes central metabolism also including the upregulation of the gad system to presumably restore the intracellular pH among others (Serra-Castelló et al., 2021b). Therefore, it may be hypothesized that the mechanisms developed by L. monocytogenes to overcome the lactate stress, which were reported to cross-protect the pathogen from HPP (Serra-Castelló et al., 2021b), could also play a role on the interaction between MAP and HPP depending on the duration of CO2 exposure before HPP. Nevertheless, further studies would be required to unravel the impact of CO2 on L. monocytogenes physiology, membrane properties, and the subsequent HPP lethality. In the case of L. sakei, similar inactivation by HPP was observed in MAP and VP samples (Fig. 1 and Table 2), which indicated that the concentration of CO2 used in MAP and/or the exposure time (1 h) were not high enough to enhance the lethality of L. sakei by HPP. On the other hand, contrary to L. monocytogenes, the MAP-exposure enhanced HPP inactivation of L. sakei in all cooked ham types, being statistically significant in standard cooked hams of both brands and in the sodium-reduced product of brand B (Table 2). Moreover, in cooked hams of brand B, the shape of the inactivation curve changed from convex (p > 1 in MAP) to concave (p < 1 in MAP-exposed) (Fig. 1). As the pH of MAP and MAP-exposed samples was not different, other factors would be responsible for the effect of MAP-exposure on HPP lethality. To the authors’ knowledge, no information is available about the impact of CO2 on the physiology of Latilactobacillus (previously Lactobacillus) genus. Nevertheless, the opposite behavior of L. monocytogenes and L. sakei to MAP exposure suggests that different CO2 stress-response mechanisms or tolerances could be involved and highlights that the HPP response is species and product dependent.
Conclusions
Sodium-reduced formulations of cooked ham can decrease the efficacy of HPP in inactivating bacteria, being their impact more relevant for L. sakei than L. monocytogenes. The cooked ham formulation used by producers stands as one of the major factors determining the efficacy of HPP and highlights the importance of applying product-oriented approaches.
Moreover, the packaging system and the time period between packaging and HPP raise as relevant factors influencing the efficacy of HPP as a lethality treatment in cooked ham with opposite impact on L. monocytogenes and L. sakei. Thus, if HPP is applied as a control measure for L. monocytogenes, cooked meat producers must pressurize their products as soon as possible after manufacturing in order to not decrease the efficacy of HPP. When HPP aims to control spoilage levels, the pressurization of the cooked meat products 1 day after manufacturing can enhance the lethality of L. sakei by HPP.
Data Availability
The data of the present study are available in response to request.
References
Anonymous. (1979). Orden de 31 de julio de 1979 por la que se establecen métodos oficiales de análisis de aceites y grasas, productos cárnicos, cereales y derivados, fertilizantes, productos fitosanitarios, productos lácteos, piensos, aguas y productos derivados de la uva. Boletin Oficial del Estado, 207, 20221-20346
Aymerich, M. T., Garriga, M., Costa, S., Monfort, J. M., & Hugas, M. (2002). Prevention of ropiness in cooked pork by bacteriocinogenic cultures. International Dairy Journal, 12(2), 239–246. https://doi.org/10.1016/S0958-6946(01)00143-1
Balamurugan, S., Ahmed, R., Chibeu, A., Gao, A., Koutchma, T., & Strange, P. (2016). Effect of salt types and concentrations on the high-pressure inactivation of Listeria monocytogenes in ground chicken. International Journal of Food Microbiology, 218, 51–56. https://doi.org/10.1016/j.ijfoodmicro.2015.11.010
Bover-Cid, S., Belletti, N., Aymerich, T., & Garriga, M. (2015). Modeling the protective effect of aw and fat content on the high pressure resistance of Listeria monocytogenes in dry-cured ham. Food Research International, 75, 194–199. https://doi.org/10.1016/j.foodres.2015.05.052
Bover-Cid, S., Serra-Castelló, C., Dalgaard, P., Garriga, M., & Jofré, A. (2019). New insights on Listeria monocytogenes growth in pressurised cooked ham: A piezo-stimulation effect enhanced by organic acids during storage. International Journal of Food Microbiology, 290(October 2018), 150–158. https://doi.org/10.1016/j.ijfoodmicro.2018.10.008
Capaldo, S. M. (2020) Evaluation of food recalls and withdrawals trends between 2014 and 2018 and risk management tools to reduce the financial impact of a recall. Major project report. Virginia Tech https://vtechworks.lib.vt.edu/handle/10919/99721. Accessed 08 July 2024
Deis, R. C., & Kearsley, M. W. (2012). Sorbitol and mannitol. In Sweeteners and Sugar Alternatives in Food Technology (pp. 331–346). https://doi.org/10.1002/9781118373941.ch15
Desmond, E. (2006). Reducing salt: A challenge for the meat industry. Meat Science, 74(1), 188–196. https://doi.org/10.1016/j.meatsci.2006.04.014
Devlieghere, F., Debevere, J., & Van Impe, J. (1998). Concentration of carbon dioxide in the water-phase as a parameter to model the effect of a modified atmosphere on microorganisms. International Journal of Food Microbiology, 43(1), 105–113. https://doi.org/10.1016/S0168-1605(98)00101-9
Devlieghere, F., Van Belle, B., & Debevere, J. (1999). Shelf life of modified atmosphere packed cooked meat products: A predictive model. International Journal of Food Microbiology, 46(1), 57–70. https://doi.org/10.1016/s0168-1605(98)00175-5
Devlieghere, F., Geeraerd, A. H., Versyck, K. J., Vandewaetere, B., Van Impe, J., & Debevere, J. (2001). Growth of Listeria monocytogenes in modified atmosphere packed cooked meat products: A predictive model. Food Microbiology, 18(1), 53–66. https://doi.org/10.1006/fmic.2000.0378
Devlieghere, F., Vermeiren, L., Bontenbal, E., Lamers, P.-P., & Debevere, J. (2009). Reducing salt intake from meat products by combined use of lactate and diacetate salts without affecting microbial stability. International Journal of Food Science and Technology, 44(2), 337–341. https://doi.org/10.1111/j.1365-2621.2008.01724.x
EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). (2021). The European Union one health 2020 zoonoses report. EFSA Journal, 19(12), 6971–7295. https://doi.org/10.2903/j.efsa.2021.6971
EFSA Biohaz Panel (EFSA Panel on Biological Hazards), Ricci, A., Allende, A., Bolton, D., Chemaly, M., Davies, R., FernándezEscámez, P. S., Girones, R., Herman, L., Koutsoumanis, K., Nørrung, B., Robertson, L., Ru, G., Sanaa, M., Simmons, M., Skandamis, P., Snary, E., Speybroeck, N., Ter Kuile, B., … Lindqvist, R. (2018). Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA Journal, 16(1), 5134–5307. https://doi.org/10.2903/j.efsa.2018.5134
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis, K., Alvarez-Ordóñez, A., Bolton, D., Bover-Cid, S., Chemaly, M., Davies, R., De Cesare, A., Herman, L., Hilbert, F., Lindqvist, R., Nauta, M., Peixe, L., Ru, G., Simmons, M., Skandamis, P., Suffredini, E., Castle, L., Crotta, M., … Allende, A. (2022). The efficacy and safety of high-pressure processing of food. EFSA Journal, 20(3), 7128–7323. https://doi.org/10.2903/j.efsa.2022.7128
FDA/USDA. (2013). Interagency risk assessment: Listeria monocytogenes in retail delicatessens. Technical report. Department of Health and Human Services, Center for Food Safety and Applied Nutrition, Food and Drug Administration and U.S. Department of Agriculture Food Safety and Inspection Service.
Francis, G. A., Scollard, J., Meally, A., Bolton, D. J., Gahan, C. G. M., Cotter, P. D., Hill, C., & O’Beirne, D. (2007). The glutamate decarboxylase acid resistance mechanism affects survival of Listeria monocytogenes LO28 in modified atmosphere-packaged foods. Journal of Applied Microbiology, 103(6), 2316–2324. https://doi.org/10.1111/j.1365-2672.2007.03466.x
FSANZ (2023) Food recall statistics. Food Stadards Austraila an New zealand. https://www.foodstandards.gov.au/industry/foodrecalls/recallstats/pages/default.aspx. Accessed 08 July 2024
Garriga, M., Aymerich, T., Costa, S., Gou, P., Monfort, J. ., & Hugas, M. (1998). Bioprotective cultures in order to prevent slime in cooked meat products. Proceedings of the 44th International Congress of Meat Science and Technology, 328–329.
Gayán, E., Condón, S., Álvarez, I., Nabakabaya, M., & Mackey, B. (2013). Effect of pressure-induced changes in the ionization equilibria of buffers on inactivation of Escherichia coli and Staphylococcus aureus by high hydrostatic pressure. Applied and Environmental Microbiology, 79(13), 4041–4047. https://doi.org/10.1128/AEM.00469-13
Grossi, A., Søltoft-Jensen, J., Knudsen, J. C., Christensen, M., & Orlien, V. (2012). Reduction of salt in pork sausages by the addition of carrot fibre or potato starch and high pressure treatment. Meat Science, 92(4), 481–489. https://doi.org/10.1016/j.meatsci.2012.05.015
Hauben, K. J., Bernaerts, K., & Michiels, C. W. (1998). Protective effect of calcium on inactivation of Escherichia coli by high hydrostatic pressure. Journal of Applied Microbiology, 85(4), 678–684. https://doi.org/10.1111/j.1365-2672.1998.00577.x
Hereu, A., Dalgaard, P., Garriga, M., Aymerich, T., & Bover-Cid, S. (2012). Modeling the high pressure inactivation kinetics of Listeria monocytogenes on RTE cooked meat products. Innovative Food Science & Emerging Technologies, 16, 305–315. https://doi.org/10.1016/j.ifset.2012.07.005
Hunt, K., Vacelet, M., & Jordan, K. (2017). Determination of Listeria monocytogenes numbers at less than 10 cfu/g. Irish Journal of Agricultural and Food Research, 56, 25–30. https://doi.org/10.1515/ijafr-2017-0004
Iulietto, M. F., Sechi, P., Borgogni, E., & Cenci-Goga, B. T. (2015). Meat spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Italian Journal of Animal Science, 14(3), 4011. https://doi.org/10.4081/ijas.2015.4011
Jydegaard-Axelsen, A.-M., HøibyPoul, E., Holmstrøm, K., Russell, N., & Knøchel, S. (2004). CO2- and anaerobiosis-induced changes in physiology and gene expression of different Listeria monocytogenes strains. Applied and Environmental Microbiology, 70(7), 4111–4117. https://doi.org/10.1128/AEM.70.7.4111-4117.2004
Jydegaard-Axelsen, A.-M., Aaes-Jørgensen, A., Granly Koch, A., Stoumann Jensen, J., & Knøchel, S. (2005). Changes in growth, rRNA content, and cell morphology of Listeria monocytogenes induced by CO2 up- and downshift. International Journal of Food Microbiology, 98(2), 145–155. https://doi.org/10.1016/j.ijfoodmicro.2004.05.019
Koseki, S., & Yamamoto, K. (2007). Water activity of bacterial suspension media unable to account for the baroprotective effect of solute concentration on the inactivation of Listeria monocytogenes by high hydrostatic pressure. International Journal of Food Microbiology, 115(1), 43–47. https://doi.org/10.1016/j.ijfoodmicro.2006.10.005
Leistner, L. (2000). Basic aspects of food preservation by hurdle technology. International Journal of Food Microbiology, 55(1–3), 181–186.
Messerli, F. H., Hofstetter, L., Syrogiannouli, L., Rexhaj, E., Siontis, G. C. M., Seiler, C., & Bangalore, S. (2021). Sodium intake, life expectancy, and all-cause mortality. European Heart Journal, 42(21), 2103–2112. https://doi.org/10.1093/eurheartj/ehaa947
MHCA (Ministry of Health and Consumer Affairs). (2005). Policy-Spanish strategy for nutrition, physical activity and prevention of obesity (NAOS). Ministry of Health and Consumer Affairs. Spanish Government. URL: https://extranet.who.int/nutrition/gina/en/node/8402 (Accessed 1st April 2022)
Molina-Höppner, A., Wolfgang, D., Vogel Rudi, F., & Gänzle Michael, G. (2004). Protective effect of sucrose and sodium chloride for Lactococcus lactis during sublethal and lethal high-pressure treatments. Applied and Environmental Microbiology, 70(4), 2013–2020. https://doi.org/10.1128/AEM.70.4.2013-2020.2004
Oxen, P., & Knorr, D. (1993). Baroprotective effects of high solute concentrations against inactivation of Rhodotorula rubra. LWT - Food Science and Technology, 26(3), 220–223. https://doi.org/10.1006/fstl.1993.1048
Patazca, E., Koutchma, T., & Balasubramaniam, V. M. (2007). Quasi-adiabatic temperature increase during high pressure processing of selected foods. Journal of Food Engineering, 80, 199–205.
R Core Team. (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/. Accessed 08 July 2024
Ruusunen, M., & Puolanne, E. (2005). Reducing sodium intake from meat products. Meat Science, 70(3), 531–541. https://doi.org/10.1016/j.meatsci.2004.07.016
Ruusunen, M., Särkkä-Tirkkonen, M., & Puolanne, E. (2001). Saltiness of coarsely ground cooked ham with reduced salt content. Agricultural and Food Science, 10(1), 27–32. https://doi.org/10.23986/afsci.5676
Serra-Castelló, C., Jofré, A., Belletti, N., Garriga, M., & Bover-Cid, S. (2021a). Modelling the piezo-protection effect exerted by lactate on the high pressure resistance of Listeria monocytogenes in cooked ham. Food Research International, 140, 110003. https://doi.org/10.1016/j.foodres.2020.110003
Serra-Castelló, C., Possas, A., Jofré, A., Garriga, M., & Bover-Cid, S. (2022). Enhanced high hydrostatic pressure lethality in acidulated raw pet food formulations was pathogen species and strain dependent. Food Microbiology, 104, 104002. https://doi.org/10.1016/j.fm.2022.104002
Serra-Castelló, C., Ferrocino, I., Jofré, A., Cocolin, L., Bover-Cid, S., & Rantsiou, K. (2021b). Unravelling the molecular mechanisms underlying the protective effect of lactate on the high-pressure resistance of Listeria monocytogenes. Biomolecules, 11(5). https://doi.org/10.3390/biom11050677
Setikaite, I., Koutchma, T., Patazca, E., & Parisi, B. (2009). Effects of water activity in model systems on high-pressure inactivation of Escherichia coli. Food and Bioprocess Technology, 2(2), 213–221. https://doi.org/10.1007/s11947-008-0069-7
Sivertsvik, M., & Jensen, J. S. (2005). Solubility and absorption rate of carbon dioxide into non-respiring foods. Part 3: Cooked meat products. Journal of Food Engineering, 70(4), 499–505. https://doi.org/10.1016/j.jfoodeng.2004.10.005
Szalai, M., Szigeti, J., Farkas, L., Varga, L., Réti, A., & Zukál, E. (2004). Effect of headspace CO2 concentration on shelf-life of cooked meat products. Acta Alimentaria, 33(2), 144–155. https://doi.org/10.1556/aalim.33.2004.2.6
Teixeira, J. S., Maier, M. B., Miller, P., Gänzle, M. G., & McMullen, L. M. (2016). The effect of growth temperature, process temperature, and sodium chloride on the high-pressure inactivation of Listeria monocytogenes on ham. European Food Research and Technology, 242(12), 2021–2029. https://doi.org/10.1007/s00217-016-2700-6
Teixeira, A., Domínguez, R., Ferreira, I., Pereira, E., Estevinho, L., Rodrigues, S., & Lorenzo, J. M. (2021). Effect of NaCl replacement by other salts on the quality of bísaro pork sausages (PGI Chouriça de Vinhais). Foods, 10(5), 961. https://doi.org/10.3390/foods10050961
van Boekel, M. A. J. S. (2021). To pool or not to pool: That is the question in microbial kinetics. International Journal of Food Microbiology, 354, 109283. https://doi.org/10.1016/j.ijfoodmicro.2021.109283
Weiss, J., Gibis, M., Schuh, V., & Salminen, H. (2010). Advances in ingredient and processing systems for meat and meat products. Meat Science, 86(1), 196–213. https://doi.org/10.1016/j.meatsci.2010.05.008
WHO (World Health Organisation). (2004). Global strategy on diet, physical activity and health. World Health Organization. https://www.who.int/publications/i/item/9241592222. (Accessed 1st April 2022)
Zhang, Y., & Cremer, P. S. (2006). Interactions between macromolecules and ions: The Hofmeister series. Current Opinion in Chemical Biology, 10(6), 658–663. https://doi.org/10.1016/j.cbpa.2006.09.020
Zwietering, M. H., Jongenburger, I., Rombouts, F. M., & Van’t Riet, K. (1990). Modeling of the bacterial growth curve. Applied and Environmental Microbiology, 56(6), 1875–1881. https://doi.org/10.1128/aem.56.6.1875-1881.1990
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the Consolidated Research Group (2017 SGR 1650 and 2021 SGR 00468) and the CERCA Programme (Generalitat de Catalunya).
Author information
Authors and Affiliations
Contributions
C.S.-C.: conceptualization, methodology, formal analysis, data curation, and writing—original draft. A.J. and S.B.-C.: conceptualization, supervision, resources and funding acquisition, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Serra-Castelló, C., Jofré, A. & Bover-Cid, S. Enhancing High-Pressure Bacterial Inactivation by Modified Atmosphere Packaging: Effect of Exposure Time and Cooked Ham Formulation. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03511-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03511-z