Abstract
The lactose intolerance—as a limiting factor for dairy milk consumption—has a high prevalence worldwide. Dairy milk and milk-derived products are major sources of multiple inorganic compounds and nutrients and thus are considered to be functional foods. β-galactosidases are able to hydrolyze lactose and are therefore widely applied for the production of lactose-free products. In addition, they are capable of the synthesis of galacto-oligosaccharides (GOSs); thus, the dairy industry has a special interest in applying them for the enrichment of dairy products with prebiotic GOSs. In this work, we studied two commercially available β-galactosidase products: Saphera 2600L and Nola Fit 5500. Both enzyme solutions contain a recombinant β-galactosidase of Bifidobacterium bifidum and have already been authorized for food industrial application, but the information about their hydrolytic and/or synthetic activities is only limited. After immobilization on chitosan beads, the enzymes were used for lactose hydrolysis and simultaneous synthesis of GOSs, by performing the reactions in pasteurized milk (skim milk). Both immobilized β-galactosidase exhibited elevated lactose hydrolysis (vmax increased from ~ 1 to ~ 4 mM/min) and GOS synthesis as compared to the free enzymes. The enzyme-coated beads were efficiently re-used at least 15 cycles; the residual lactose concentration was < 2 mg/ml after each cycle. After treatment, GOSs were present in ≤ 9% of the total sugar content, indicating that the prepared low-lactose milks were enriched in prebiotic GOSs. The application of immobilized Saphera 2600L and Nola Fit 5500 β-galactosidases may be implemented for the large-scale production of GOS-enriched low-lactose milk.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactose (LAC) is one of the main components of milk, a slightly sweet disaccharide consisting of glucose (GLC) and galactose (GAL). The concentration of LAC in milk is at 4.5–5%, depending on the dairying species (Ratajczak et al., 2021). LAC principally is an important carbon and energy source for the human cells, and it can be utilized by the intestinal microbiome, as well. Digestion of LAC by lactase (β-galactosidase) releases free GLC and GAL which monosaccharides can be absorbed in the digestive system.
Global statistic estimated that about 65–75% of the adult population worldwide has different phases of lactose intolerance (Deng et al., 2015; Storhaug et al., 2017). In lactose intolerance, deficiency of the lactase causes insufficient digestion of LAC that may be correlated with multiple gastrointestinal and extra-intestinal symptoms. Consequently, LAC may cause various problems including those of gastrointestinal health, such as abdominal pain, bloating, elevated gas production, nausea, vomiting, or diarrhea, depending on the amount of the produced lactase and the amount of consumed lactose (Lomer et al., 2007). The presence of unabsorbed lactose increases the osmotic force in the bowel lumen, and the fluid influx results in osmotic diarrhea. In addition, the unabsorbed lactose is utilized by the microbial consortia, the fermentation results in gas production, and the produced lactic acid causes acidification which may impair the functions of digestive enzymes.
People who suffer from the above-described symptoms generally tend to avoid consumption of fresh milk and milk products, but omitting them from the diet may lead to various deficiency diseases (Ratajczak et al., 2021). Therefore, there is an increasing pressure on the food industry to produce a wide selection of lactose-free or low-lactose dairy products which can be consumed by people suffering from lactose intolerance, as well. The food industry—in cooperation with researchers—has searched for possible ways to meet the expectations, needs, and requests of customers (Sharp et al., 2021). The products which can be incorporated into the diets of lactose intolerants include fermented milk products (such as kefir, yoghurt) containing a reduced level of lactose. Nevertheless, there is a grooving interest in functional foods, which—beyond their nutritional value—offer health benefits, e.g., by containing supplements or other additional ingredients. Multiple products are targets of the dairy industry to be enriched in ingredients of beneficial properties such as vitamins, syn-, post-, pro-, or prebiotics; the fortified products may have a positive effect on health and help to reduce the risks for the development of diseases (Al-Sheraji et al., 2013; Rudke et al., 2023).
The enzyme β-galactosidase has been widely used in the dairy industry for the hydrolysis of LAC into GLC and GAL (Nijpels, 1981) which is essential for the production of low-lactose dairy products that are edible for lactose-intolerant individuals. Due to the increasing market potential of lactose-free dairy products (Kocabaş et al., 2022), several β-galactosidases have been commercialized (Fischer & Kleinschmidt, 2018; Annapure & Gaur, 2022). The potential industrial applications of free (Botvynko et al., 2019; Füreder et al., 2020) and immobilized forms (Carrara & Rubiolo, 1994; Neto et al., 2021; Nguyen et al., 2019) have also been studied, but in many cases, the reactions were investigated in buffer(ed) solutions rather than in the complex biological environment of the milk.
In the past few decades, several studies focused on the advantages and disadvantages of the usage of free enzymes, including their application to achieve total LAC hydrolysis (Anisha, 2017; Vera et al., 2020). In addition, several attempts have been made to study β-galactosidases as biocatalysts of galacto-oligosaccharide (GOS) synthesis via trans-galactosylation in which enzymatic reaction lactose-derived compounds are synthesized by the lactase during LAC hydrolysis (Grosová et al., 2008; Fischer & Kleinschmidt, 2018; Mano et al., 2019). During the hydrolysis of the β(1–4) linkage of lactose, the glucose is released, and a covalent enzyme-galactose intermediate is formed. After this, the galactosyl moiety can be transferred to the hydroxyl group of an acceptor molecule. In the case of hydrolysis, the galactosyl acceptor is water, resulting in the release of free galactose. If the acceptor molecule is an alternative molecule, such as an another carbohydrate, transgalactosylation occurs. The acceptor of galactosyl transfer reaction can be glucose, galactose, lactose, or a GOS molecule, as well (Gosling et al., 2010). Due to their ability for LAC hydrolysis as well as GOS synthesis, β-galactosidases are considered valuable tools for the production of low-lactose dairy products containing GOSs (Iqbal et al., 2010; Urrutia et al., 2013; Nath et al., 2016).
Together with other various carbohydrates—such as lactulose, inulin, and fructo-, isomalto-, xylo-, and soybean oligosaccharides—GOSs are functional food ingredients and belong to the group of molecules that are recognized as prebiotics (Dominguez et al., 2014; Souza et al., 2022; Rudke et al., 2023). GOSs are non-digestible oligosaccharides which resist most digestive enzymes and can stimulate the growth and/or activity of the bacteria being members of the microbial consortia in the colon, including bifidobacteria (Panesar et al., 2006; Roberfroid et al., 2010; O'Callaghan & van Sinderen et al., 2016; Hidalgo-Cantabrana et al., 2017; Zhang et al., 2023). Several studies revealed the beneficial effects of GOSs on health (Sanders et al., 2019; Sangwan et al., 2015) and focused on the access to products with high GOS content (Rodriguez-Colinas et al., 2014; González-Delgado et al., 2018; Urrutia et al., 2018).
In the dairy industry, the LAC removal or the reduction of its level in milk is carried out by using free enzymes; the main goal of the enzymatic treatment is to produce lactose-free milk of milk-derived products that contain LAC in < 1 mg/ml concentration. But, the final products are absent from GOSs or contain these prebiotics only in low concentration due to the uncontrolled nature of GOS synthesis and hydrolysis under these conditions. During the complex reaction, the β-galactosidase hydrolyzes the LAC and synthesizes the GOSs, but after running out of the LAC, the produced GOSs also become substrates of the enzyme (Otieno, 2010). In the industrial technology, the commercially available lactose-free milks are commonly produced by supplementing the milk with soluble β-galactosidase (Dekker et al., 2019). Consequently, after packaging, transportation, and storage, the products still contain the free β-galactosidase which has already been hydrolyzed not only LAC but also GOSs.
A better control of the simultaneous LAC hydrolysis and GOS synthesis may be facilitated by the immobilization of β-galactosidases, which may offer an alternative for the use of free enzymes (Ohmiya et al., 1975, 1977; Panesar et al., 2010; de Souza et al., 2022). The immobilized enzymes enable a more precise control of the complex transgalactosylation reactions. In addition, the enzymes can be easily removed from the milk at an optimal time point (e.g., by filtration); consequently, the products—finally reaching the consumers—are absent from the lactase enzymes which have been added previously for removal of LAC. Several solid supports, such as biopolymer-based beads (Bedade et al., 2019; Flores et al., 2019; Kurayama et al., 2020), silica gels (González-Delgado et al., 2018), hydrogels (Wolf et al., 2021), magnetic nanoparticles (Nguyen et al., 2019), and inorganic particles (Husain et al., 2011; Khan et al., 2019), have been developed and tested for enzyme immobilization so far. In addition, it has also been reviewed that not only solid supports but liposomes may also be good candidates to carry and deliver enzymes such as β-galactosidases (Mohammadi et al., 2021).
Based on our previous experiences (Bodnár et al., 2005; Polyák et al., 2014), chitosan (CH) biopolymer was chosen to prepare beads for enzyme immobilization. CH is a renewable, basic linear biomaterial, containing β-[1 → 4]-linked 2-acetamido-2-deoxy-D-glucopyranose and 2-amino-2-deoxy-D-glucopyranose units. CH is prepared from chitin which is the second most abundant biopolymer in nature (Hajdu et al., 2008). It has a special set of beneficial properties including low or non-toxicity, biocompatibility, biodegradability, low immunogenicity, and antibacterial properties. Due to the available amino groups of CH, enzymes can be immobilized efficiently on the CH surface by using different methodologies, including adsorption (Wolf et al., 2019), encapsulation, or covalent bonding (Panesar et al., 2010; Pan et al., 2009); the covalent immobilization can prevent the dissociation of the enzymes from the support.
In this study, we used two different β-galactosidase products, the Saphera 2600L and Nola Fit 5500; these full names are abbreviated in the text as Saphera and Nola Fit, respectively. Both enzyme solutions have international food industrial licenses and were approved for dairy industrial application (including the production of a wide variety of dairy products, such as milk, cheese, cream, and drinks). These products are supplied as liquid solutions containing highly purified enzymes; therefore, their application can easily be standardized. Both the Saphera 2600L and the Nola Fit 5500 enzyme solutions contain a wide spectrum ß-galactosidase of Bifidobacterium bifidum (EC: 3.2.1.23) produced in Bacillus licheniformis strain. These products represent a new type of lactases, as they contain a recombinantly produced enzyme which has a broader pH tolerance. The lactase of B. bifidum belongs to the enzymes that are most widely applied for the processing of milk or other dairy liquids as well as are enzymatic tools of GOS synthesis (Goulas et al., 2007; Fischer & Kleinschmidt, 2018). Although multiple studies targeted the investigation of the B. bifidum galactosidase (Botvynko et al., 2019; Füreder et al., 2020; Schmidt et al., 2020; Majore & Ciproviča, 2020; Czyzewska & Trusek, 2021; Popescu et al., 2021), the information about the hydrolytic and/or synthetic activities of Saphera and Nola Fit in milk are still limited; most information was obtained by studying the enzyme activities in buffers rather than in milk or any other dairy liquids.
In this work, we aimed to optimize conditions for the production of GOS-enriched milk by using commercially available β-galactosidases that are covalently immobilized on CH beads. Such conditions were tested which were considered to be potentially compatible with industrial applications, such as performing the enzymatic reactions in homogenized pasteurized milk. We studied both LAC hydrolysis and GOS synthesis reactions as well as the characteristics of the free and immobilized enzymes, and the operational and thermostabilities of the immobilized enzymes were also determined. The investigation of the reaction parameters—which may be relevant from a technological point of view—was considered to provide valuable information for the industrial implementation of the protocols which can be used for the production of lactose-free milk enriched in GOS.
Materials and Methods
Materials
Chitosan (CH) (named 90/1000 with a degree of deacetylation 90%) was purchased from Heppe Medical Chitosan GmbH (Germany). Potassium phosphate monobasic, potassium phosphate dibasic, acetic acid, sodium hydroxide, and glutaraldehyde (GA, 50% (v/v)) were obtained from Avantor Inc. (Hungary). O-nitrophenyl-β-D-galactopyranoside (ONPG; #34055) was from Thermo Fisher Scientific (Waltham, MA, USA). Megazyme LAC assay kit was purchased from Noack Magyarország Kft. (Hungary). All chemicals were of analytical grade.
D(+)-glucose (Sigma-Aldrich, G8270), D(+)-galactose (BioChemica, A11310100), D(+)-sucrose (internal standard) (VWR, 27483.294), and lactose monohydrate (VWR, 10,039–26-6) were used as standards for HPLC-based analyses. Carrez solution I and II reagents (purchased from VWR Chemicals, Hungary) (Carrez I: Cat.No. 85733.290; Carrez II: Cat. no. 5056.1000) were applied for the determination of sugars. Allolactose, galactobiose, and 6′-galactosyllactose (Megazyme) were obtained from Noack Magyarország Kft. (Hungary). GOSs (OG32134) were ordered from Biosynth Carbosynth (Slovakia). HPLC-grade acetonitrile was purchased from Thomasker (Hungary). High-purity water was prepared using a Millipore system.
We used homogenized pasteurized milk (fat content: 1.5%) that was randomly selected from milk being produced and commercially available in Hungary. The initial LAC concentration of milk (which varied between 50 and 55 mg/ml) was determined by a Megazyme LAC assay kit (Megazyme) and an HPLC-based method as well.
Enzymes
Two commercially available enzyme solutions were applied: (i) Nola Fit 5500 (with the activity of 5500 Bifido lactase unit (BLU·g−1); Chr.HANSEN, Denmark) and (ii) Saphera 2600L (with the activity of 2600 lactase activity units (B-standard)/g (LAU(B)·g−1); Novozymes, Switzerland). Both solutions contain a purified β-galactosidase of Bifidobacterium bifidum which was produced by submerged fermentation using a selected strain of Bacillus licheniformis. The commercial enzyme solutions were used without any further purification; they were stored according to the manufacturer’s instructions within the 0–8 °C temperature range. For testing stability, stocks of the solutions were stored at − 20 °C as well.
SDS-PAGE Analysis
Both Saphera and Nola Fit solutions were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Walker, 1996). The enzyme solutions were 100-fold diluted with distilled water, supplemented with sample buffer containing SDS and β-mercaptoethanol, followed by denaturation at 95 °C for 10 min. Fourteen percent polyacrylamide gel was used for electrophoretic separation (120 V, ~ 90 min), using Page Ruler Prestained Protein Ladder (Thermo Fisher Scientific, #26619) as molecular weight standard. The gels were stained with Coomassie Brilliant Blue R-250 dye, followed by imaging of the gels by using AlphaImager HP (Protein Simple, USA) imaging system.
The band intensities were determined by densitometric analysis of the detected bands by using GelAnalyzer 19.1 software (GelAnalyzer 19.1 by István Lázár Jr., PhD and István Lázár Sr., PhD, CSc; www.gelanalyzer.com).
Preparation of Chitosan Beads
CH beads were prepared by a precipitation method described previously (Flores et al., 2019; Klein et al., 2016) with some modifications. CH was dissolved in 3% (v/v) acetic acid aqueous solution to achieve a 2% (w/w) final polymer concentration. After mixing the CH solution for 12 h, it was sonicated for 2 min to remove the air bubbles and then poured into a syringe (needle diameter: 0.45 mm). The solution was allowed to drop into 1 M NaOH solution while continuously stirring. For the hardening of chitosan beads, the solution was mixed for 4 h at room temperature. Finally, the CH beads were washed with distilled water and 0.1 M phosphate buffer (pH 7.0). Beads having uniform shape and size were obtained and stored in phosphate buffer at 4 °C until used.
Activation of Chitosan Beads
Bead activation was performed by using a series of 0.1 M potassium-phosphate buffer (pH 7.0) containing GA in 0.5–5% (v/v) concentration range while slowly stirring at room temperature (using a magnetic stirrer, 80–100 rpm). Reaction time varied between 30 min and 6 h. The activated CH beads were washed with 0.1 M phosphate buffer (pH 7.0) solution to remove the excess GA. The statistical differences between the specific activities were determined by using the freely available QuickCalcs online tool of GraphPad (https://www.graphpad.com/quickcalcs/ttest1/).
Immobilization of β-galactosidase on Chitosan Beads
Immobilization reactions were carried out via slow continuous stirring of the CH beads with the β-galactosidase enzyme solutions (Saphera or Nola Fit) overnight at room temperature, by using a magnetic stirrer (80–100 rpm). If it was necessary, the enzyme solutions were diluted with 0.1 M potassium-phosphate buffer (pH 7.0) to achieve different final concentrations while optimizing the conditions of immobilization. After immobilization, successive washing steps were applied using 0.1 M phosphate-potassium buffer (pH 7.0) to remove the excess enzyme, until no enzymatic activity was detected in the supernatant (see the details of enzyme activity assays below).
Determination of Enzymatic Activity
Enzymatic activities of the free and immobilized β-galactosidases were determined by using ONPG as substrate, based on the manufacturer’s instructions. For the free enzymes, the diluted enzyme solutions (150 µl) were added to ONPG substrate (5 mM, freshly dissolved in 50 mM sodium phosphate solution, pH 7.0) in equal volume (final concentration of ONPG was 2.5 mM). In the case of the immobilized enzymes, 150 µl ONPG solution (5 mM in 50 mM sodium phosphate solution, pH 7.0) was added to five randomly selected CH beads (coated with the immobilized enzymes). Afterwards, the reaction mixtures were incubated for 10 min at 37 °C, and thereafter, the reactions were immediately stopped by the addition of 1 M sodium carbonate solution (150 µl). The absorbance of the released O-nitrophenol was measured spectrophotometrically at 420 nm wavelength using a Synergy H1 reader (BioTek Instruments, Hungary). The rate of product formation (ΔOD/min) was determined based on the absorbance values, and it was used to calculate relative activities (%).
Determination of the Yield of Immobilization
The yield of immobilization (%) was determined based on the activity that was calculated from the absorbance of supernatant before and after immobilization, using the following equation:
Optimization of the Reaction Conditions for Enzyme Immobilization
Optimization of reaction conditions was performed in 0.1 M sodium phosphate solution at room temperature. We have investigated the effects of GA concentration, reaction time, and enzyme concentration on the efficacy of immobilization. The reaction time of GA activation ranged from 30 min to 6 h, while the GA concentration was applied in the 0.75–5% (v/v) concentration range. The final concentrations of the commercial β-galactosidase solutions were also changed; they varied between 0.75 and 10% (v/v). The optimal values of independent factors were selected based on the enzymatic activity of β-galactosidase immobilized on CH beads measured by ONPG as substrate described above.
Quantitative Limits of Activation and Immobilization
To determine the quantitative limits of bead activation and enzyme immobilization, the reactions contained the CH beads in different final concentrations. The activation and enzyme immobilization reactions were performed in 0.1 M sodium phosphate solution, as described above. We applied standard reaction conditions in the different reactions, i.e., the GA activation reagent and the β-galactosidase enzyme solutions (Saphera or Nola Fit) were applied in the same concentration. The final sample volumes were also constant while the CH bead concentrations ranged from 25 to 100 bead/ml in the mixtures.
The bead activation and enzyme immobilization were followed by measuring the enzyme activities of the coated beads (CH-Saphera and CH-Nola Fit). The activities were determined in parallel by using 5–5 randomly selected beads from each sample; the ONPG substrate was applied as described above. The activity obtained for the 25 CH bead/ml sample was considered to be 100% and used as a reference.
Thermal Stability
Thermal stabilities of the free and immobilized enzymes were studied in phosphate buffer (0.1 M, pH 6.5) at 50 °C. The samples were incubated in a thermostatically controlled water bath for 8 h). After incubation, the samples were placed onto ice in order to stop the inactivation reaction immediately. The residual enzyme activity was determined based on the method described above, using ONPG as substrate. The measurements were performed in triplicates.
Operational Stability
Operational stabilities of the immobilized enzymes were determined by incubating the CH beads in homogenized pasteurized milk in batch cycles. The CH beads were applied in 20 bead/ml milk concentration; the reaction mixtures were incubated while continuously rotating at 1000 rpm for 2 h at 37 °C. Samples were withdrawn periodically, and residual LAC concentration of milk supernatant was determined by Megazyme LAC assay kit (Megazyme) and an HPLC-based method as well. After each cycle, CH beads were removed from the milk by simple filtration (using filter paper), washed with MQ water, and then used repeatedly in a new reaction cycle at the same conditions described above, without any further activation or modification (measured in duplicates).
Lactose Hydrolysis and Enzymatic Synthesis of Galacto-oligosaccharides
To study the simultaneous LAC hydrolysis and GOS synthesis, the enzymatic reactions were performed in homogenized pasteurized milk without any adjusted buffered conditions. For this study, the enzyme-coated CH beads were applied in 20 bead/ml milk concentration; the reaction mixtures were rotated on a shaker at 1000 rpm at 37 °C for 2 h while monitoring the reactions at different time points. Then, the CH beads were removed, and milk samples were collected without any further treatment or filtration. For comparison, LAC hydrolysis and GOS synthesis reactions were investigated using enzyme solutions containing the free enzyme, as well. Finally, the treated milks were boiled for 5 min. Concentrations of the free enzymes were set based on the manufacturer’s instructions. LAC and GOS concentrations were determined by HPLC-based methods described below.
Determination of Kinetic Parameters
Kinetic parameters (KM and vmax) of the free and immobilized enzyme were determined in phosphate buffer using ONPG as substrate (in the 0.25–25 mM final concentration range), using the protocol described above. After determination of the initial reaction velocity values, the data points were fitted to the Michaelis-Menten equation to determine the KM and vmax of the free and immobilized enzymes. The calculations were performed by using the Solver extension module of Microsoft Excel (Microsoft Office).
HPLC-Based Measurements
Products of transgalactosylation reactions (GOSs) and LAC were analyzed based on a method published previously (Manzi & Pizzoferrato, 2013) with some modifications. The characterization of sugar pattern and GOS formation was carried out using an HPLC system (Waters e2695 Separations Module) equipped with a refractive index detector (Waters 2414). Analysis was performed by using XBridge BEH Amide column (Waters, 4.6 × 250 mm, 5 µm) and guard column (Waters, 3.9 mm × 5 mm, 5 µm). Briefly, 25 µL of the solution was injected into the mobile phase (acetonitrile to pure water in a 7:3 ratio) at 0.80 ml/min flow rate. The temperatures of the column and the detector were also maintained at 30 °C. The concentration of the saccharides was calculated using calibration curves; the stock solutions of saccharides (dissolved in water) ranged from 0.5 to 50 mg/ml final concentrations. The plotted peak areas of HPLC chromatograms were considered to be directly proportional to the sugar concentrations.
The milk samples were treated based on a previously described protocol (Manzi & Pizzoferrato, 2013) with slight modifications. Twenty microliters of internal standard sucrose (300 mg/ml) was added to 1 ml of milk sample, followed by sequential addition of Carrez-I and Carrez-II reagents (30 µl of each) and vortexing for 2 min. Then, the extraction was performed by the addition of 600 µl of acetonitrile, vortexing for 2 min, and incubation for 20 min at room temperature. Finally, the samples were centrifuged at 10,000 rpm for 10 min at 4 °C (Eppendorf 5424R centrifuge equipped with FA-45-24-11 rotor). Before HPLC injection, the supernatants were filtered through a 0.22-µm polyethersulfone (PES) filter. Twenty-five microliters of the clear filtrates was injected into the HPLC system.
GOS Analysis by Mass Spectrometry
For mass spectrometry (MS) measurements, a Waters 2695 separation module equipped with a thermostable autosampler (5 °C) and a column module (35 °C) (each from Waters, Milford, MA, USA) was applied. All the measurements were carried out on an XBridge BEH HILIC Column (130 Å, 5 µm, 4.6 mm × 250 mm) with the isocratic method (70% acetonitrile, 30% H2O); the flow rate was 0.8 mL/min. Twenty microliters was injected from each sample. A MicroTOF-Q type Qq-TOF MS instrument (Bruker Daltoniks, Bremen, Germany) equipped with an electrospray ionization (ESI) ion source was applied in positive ion mode for MS-based detection. The spray voltage was 4 kV. N2 was used as nebulizer gas (1.8 bar) and drying gas (7 L/min, 200 °C). The spectra were calibrated externally by the ESI-tune-mix from Bruker. Extracted samples were diluted 20 times with the eluent to avoid too much adduct ions in spectra.
Sample Preparation for Lactase Analysis by Mass Spectrometry
After the separation of Saphera 2600L and Nola Fit 5500 solutions by SDS-PAGE, the protein bands were excised from the gel and subjected to in-gel trypsin digestion. Sample destaining was performed by using a 1:1 ratio of 25 mM ammonium bicarbonate (pH 8.5) and 50% acetonitrile; the samples were further reduced with 20 mM dithiothreitol (Bio-Rad, Hercules, CA, USA) for 1 h at 56 °C, followed by alkylation with 55 mM iodoacetamide (Bio-Rad, Hercules, CA, USA) for 45 min at room temperature in the dark. Trypsin digestion was performed overnight at 37 °C using stabilized MS-grade TPCK-treated bovine trypsin (ABSciex, Framingham, MA, USA). The digested peptides were extracted and dried in a speed-vac at room temperature (Thermo Fischer Scientific). The peptides were re-dissolved in 10 μL 1% formic acid (VWR Ltd., Radnor, PA, USA) before LC-MS/MS analysis.
Liquid Chromatography-Mass Spectrometry Analysis
Peptides were separated in a 180 min water/acetonitrile gradient using an Easy nLC 1200 nano UPLC (Thermo Scientific, Waltham, MA, USA). The peptide mixtures were desalted by an ACQUITY UPLC Symmetry C18 trap column (20 mm × 180 µm, 5 μm particle size, 100 Å pore size; Waters, Milford, MA, USA), followed by separation in Acclaim PepMap RSLC C18 analytical columns (150 mm × 50 μm, 2 μm particle size, 100 Å pore size, Thermo Fischer Scientific). The chromatographic separation was performed using a gradient of 5–7% solvent B over 5 min, followed by a rise to 15% of solvent B over 50 min, and then to 35% solvent B over 60 min. Thereafter, solvent B was increased to 40% over 28 min and then to 85% over 5 min, followed by a 10 min rise to 85% of solvent B, after which the system returned to 5% solvent B in 1 min for a 16 min hold-on. Solvent A was 0.1% formic acid in LC water (Sigma, St. Louis, MO, USA); solvent B was 95% acetonitrile (Sigma, St. Louis, MO, USA) containing 0.1% formic acid. The flow rate was set to 300 nL/min.
Data-dependent acquisition experiments were carried out on an Orbitrap Fusion mass spectrometer (Thermo Fischer Scientific). The 14 most abundant multiply-charged positive ions were selected from each survey MS scan, using a scan range of 350–1600 m/z for MS/MS analyses (Orbitrap analyzer resolution: 60,000, AGC target: 4.0 × 105, acquired in profile mode). Collision-induced dissociation (CID) fragmentation was performed in the linear ion trap with 35% normalized collision energy (AGC target: 2.0 × 103, acquired in centroid mode). Dynamic exclusion was enabled during the cycles (exclusion time: 45 s).
Protein Identification and Data Evaluation
The protein identification was carried out based on the acquired LC-MS/MS data by using MaxQuant 1.6.2.10 software (Cox & Mann, 2008) and searching for the B. bifidum UniProt database (release: June 2020; 30,529 sequence entries) and the contaminant database. Cys carbamidomethylation, Met oxidation, and N-terminal acetylation were set as variable modifications. A maximum of two missed cleavage sites was allowed. Results were imported into Scaffold 5.0.1 software (Proteome Software Inc., Portland, OR, USA). Proteins were accepted with at least 3 identified peptides using a 1% protein false discovery rate (FDR) and 95% peptide probability thresholds.
Results
Investigation of the β-galactosidase Enzyme Solutions
The Saphera 2600L and Nola Fit 5500 solutions were analyzed by SDS-PAGE in order to obtain information about their overall protein composition (Fig. 1A). The gel images showed high purity for both products, and the pattern of bands was found to be highly comparable. The most intensive bands were detected at a higher molecular weight range (~ 100–150 kDa); the other bands had much lower density. The band of the highest molecular weight (~ 130 kDa) was considered to be the B. bifidum β-galactosidase, and the descriptions of the products do not include such information about the recombinant protein. The other intense bands were supposed to potentially correspond to the degradation products of the full-length protein (Fig. 1A). In order to confirm this, the bands were excised from the gel and subjected to protein identification by mass spectrometry.
Investigation of the enzyme solutions. A A representative gel image shows the separation of Saphera 2600L and Nola Fit 5500 solutions by SDS-PAGE. The enzyme solutions were stored at 4 or − 20 °C; the solution which was not stored for 6 months was considered to be a reference. The 100-fold diluted solutions were analyzed by SDS-PAGE. The continuous arrow indicates the band which was supposed to contain the full-length recombinant β-galactosidase (Band130 kDa), while the dashed arrows show such lower molecular weight bands (Bands100–130 kDa) which were considered to be shorter enzyme forms, i.e., degradation products. The bands shown by the dashed arrows were excised together from the gel and subjected to protein identification by MS. B The relative band intensities were determined based on the densitometry of the gels, by comparing the highest molecular weight band’s intensity (Band130 kDa) and the overall intensities of all detected bands. The relative band intensity values are presented as average ± SD (n = 3). C The hydrolytic activities of the enzyme solutions (stored at 4 or − 20 °C for 6 months or not) were determined by using ONPG as substrate. The ΔOD/min are presented as average ± SD (n = 2)
We carried out LC-MS/MS-based protein identification in order to determine the protein compositions of the bands (Fig. 1A). Each studied band of Saphera and Nola Fit enzyme solutions (Band130 kDa and Bands100−130 kDa) contained the β-galactosidase of B. bifidum. Although, the LPXTG-motif cell wall anchor domain protein of B. bifidum was also identified in each band, the alignment of its sequence (UniProt ID: E4V8A7_BIFBI) to that of the β-galactosidase (UniProt ID: 0A0H2PBW7_BIFBI) revealed that the sequences are almost fully identical (> 99.5% sequence identity). This was in agreement with the expectations because the β-galactosidases were recombinantly produced in B. licheniformis expression strain. The analysis of bands with lower molecular weights (Bands100−130 kDa) revealed the fragments of the above-mentioned proteins and some contaminants from the cell culture such as inter-alpha (globulin) inhibitor H4 (Bos taurus), alpha-2-HS-glycoprotein precursor (Bos taurus), complement C3 precursor (Bos taurus) and keratin. B. licheniformis proteins were not identified in any of the studied bands. The detailed results of the identification with the identified peptides can be found in Table S1. The recorded spectra were uploaded to the MassIVE database and are publicly available at ftp://MSV000092352@massive.ucsd.edu.
The appearance of multiple intensive bands on the gel images (Fig. 1A) implied that the recombinant β-galactosidases might potentially undergo degradation while storing the enzyme solutions. In order to test whether the band intensities change after storage, the enzyme solution was stored either at 4 °C or − 20 °C, followed by electrophoresis. Besides determining the relative band intensities, the activities of the enzyme solutions were also measured in order to determine whether a putative degradation may cause a decrease in the enzyme activity. The β-galactosidase activities were measured by using ONPG as substrate, and the results of the SDS-PAGE analyses and enzymatic assays were correlated.
The relative amounts of the highest molecular weight proteins did not show considerable change after storage, indicating high overall stability for the enzyme solutions (Fig. 1B). In accordance with the fact that the SDS-PAGE analysis revealed no enzyme degradation, the measured enzyme activities were also comparable, the enzyme solutions stored at − 20 °C exhibited slightly higher activity as compared to those stored at 4 °C, but none of them lost their activity (Fig. 1C).
Formation of CH Beads
CH biopolymer was used for the formation of macroparticular beads; the particles were prepared by dropwise addition of the CH biopolymer solution to the precipitant using a syringe needle. The prepared CH macroparticles had a highly similar size; the average diameter was ~ 2 mm, and the beads were found to have uniform shape (Fig. 2).
Optimization of the Reaction Conditions for Enzyme Immobilization
In this work, we intended to use two commercially available enzyme solutions, namely Saphera 2600L and Nola Fit 5500, containing a recombinant β-galactosidase of B. bifidum produced by B. licheniformis. The β-galactosidases which were immobilized on CH beads are referred to as CH-Saphera and CH-Nola Fit, in order to differentiate them from the free (non-immobilized) enzymes.
Nola Fit was chosen to determine optimal conditions for enzyme immobilization; both concentration of GA and activation time were investigated at constant enzyme concentration (5% (v/v)). The reaction time varied from 0.5 to 6 h while the applied GA concentration ranged from 0.75 to 2.5% (v/v) (Table 1).
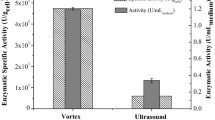
The specific activities (normalized to the weight of CH) showed no considerable differences at low GA concentration while increasing the reaction time, but the activity showed a remarkable decrease if the GA was applied at a higher concentration. The lowest activities were obtained for those samples which were incubated for 6 h at 1.25 or 2.5% (v/v) GA concentration. The activity at the same time point was considerably higher at the lowest tested GA concentration (Table 1). The difference between the specific activities obtained at 0.75 or 1.25% (v/v) GA concentrations was found to be statistically significant (p = 0.017). The specific activities were highly comparable for all GA concentrations at the shortest tested reaction time; therefore, 30-min incubation was chosen for the downstream experiments.
In the next step, both the GA and enzyme were applied in different concentrations (in 0.75–5% (v/v) and 0.75–10% (v/v) concentration range, respectively), and different GA to enzyme ratios were also tested. We found that the chitosan beads were damaged at ≥ 5% (v/v) GA concentration; therefore, the GA was then applied in the 0.75–2.5% (v/v) concentration range. The hydrolytic activities of the enzyme-coated beads were found to be proportional to their concentration; the increase of the initial concentration resulted in the increase of the β-galactosidase activity. But, in these cases, the ionic immobilization became increasingly important besides the covalent immobilization, and it must have been evaded. Due to these and by considering cost-efficiency, the enzymes were also applied in concentration ranging from 0.75 and 2.5% (v/v).
Similar but slightly different reaction conditions were required for the most effective immobilization of Saphera and Nola Fit. Both enzymes required relatively lower GA concentration in order to obtain the highest enzymatic activity, which was 1.25 and 0.75% (v/v) for Saphera and Nola Fit, respectively. Similar concentrations resulted in the highest immobilized activity on CH beads; it was 1.25% (v/v) for Saphera and 2.5% (v/v) for Nola Fit. Deviation from these specified parameters resulted in the decrease in enzyme immobilization’s efficacy. Using optimal conditions, the yield of immobilization on CH beads was ≥ 99%.
Quantitative Limits of Activation and Immobilization
Using optimal reaction conditions is essential for successful immobilization, which is in correlation with the costs of the practical applications. The quantitative limits of enzyme immobilization were investigated by using different numbers of CH beads for activation in standard reaction conditions (at constant final reaction volume as well as activation reagent (GA) and enzyme concentrations). The reaction mixtures contained the CH beads to be activated in increasing final concentrations (25–100 bead/ml). After activation and coating of the beads, the immobilization efficacy was determined by measuring the activities of CH-Saphera and CH-Nola Fit enzymes by using ONPG as substrate (Fig. 3). The activities obtained for the reference samples—beads activated in 25 beads/ml concentration—were considered to be 100%; all other activities were determined as a function of these values (different reference samples were applied for CH-Saphera and CH-Nola Fit).
Dependence of the relative activities of immobilized β-galactosidases on the number of the CH beads. The CH beads were applied for activation and immobilization in different final concentrations while using constant conditions (reaction volume, enzyme, and activator concentration). The β-galactosidase activity measurements were performed by using ONPG as substrate. The values obtained for the CH-Saphera and CH-Nola Fit enzymes are shown with dark and light gray colors, respectively. The reactions were performed at 0.75:1.25% (v/v) GA to Saphera and 1.25:2.5% (v/v) GA to Nola Fit ratios. Number of beads varied between 25 and 100 per ml. All experiments were performed in triplicates; the values are presented as mean ± SD
We observed no correlation between the number of beads and the corresponding enzyme activities (Fig. 3). The behaviors of the CH-Saphera and CH-Nola Fit enzymes were highly similar; the highest activities were measured in the 25–50 bead/ml range. The increase in bead concentration caused no increase in the relative activities; rather, a slightly decreased activity was measured for both enzymes.
Thermal Stability
According to the product information of the manufacturers, the temperature optima of the Saphera and Nola Fit β-galactosidase enzymes are also between 35 and 50 °C, while both of them can be inactivated at > 70 °C temperature. Although, the recommended temperatures may be optimal for LAC hydrolysis in this temperature range, the abilities of the immobilized enzymes may be potentially different from those of the free enzymes due to the beneficial effect of the immobilization on thermal stability (Flores et al., 2019; Hackenhaar et al., 2021; Lima et al., 2021). Therefore, we compared the thermal stabilities by performing the reactions within the optimal range and found that the immobilized enzymes exhibit higher thermal stability at 50 °C than the free enzymes.
CH-Saphera and CH-Nola Fit also exhibited higher hydrolytic activity as compared to their free forms (Fig. 4), indicating that the immobilization on CH beads improves thermostability of the studied β-galactosidases, at least at 50 °C.
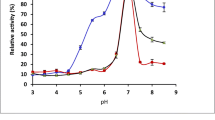
Thermal stabilities of the free and immobilized β-galactosidases at 50 °C. The activity measurements were performed by using an ONPG substrate. The relative activities were plotted as a function of time, non-linear regression lines were fitted to the data points. Free β-galactosidase: Saphera (□) and Nola Fit (∆); immobilized β-galactosidase: CH-Saphera (◼) and CH-Nola Fit (▲). All experiments were performed in triplicates, the values are presented as mean ± SD
The residual activities of the CH-Saphera and CH-Nola Fit enzymes at 50 °C were higher than 45% and 35%, respectively. Nevertheless, the activity of free enzymes was lower than 20% after 2 h of incubation. After 4 h of incubation, the free enzymes have lost 95% of their activity while the immobilized preparations exhibited more than 20% residual activity. Based on the non-linear regression analysis, we determined the incubation times where the relative activities were half-maximal. The free enzymes lost > 50% of their activity within 1 h of incubation (Fig. 4), indicating approximately threefold higher half-life for the immobilized enzymes (Saphera: 0.8 h, CH-Saphera: 2.4 h, Nola Fit: 0.6 h, and CH-Nola-Fit: 1.7 h).
Kinetic Parameters
In order to determine the effects of enzyme immobilization on catalytic properties, the kinetic parameters (KM and vmax) were determined for the free and immobilized enzymes, using ONPG as substrate. Highly similar KM values were calculated for Saphera and Nola Fit enzymes, but the immobilization remarkably increased KM; it was 8.9- and 7.4-fold higher for Saphera and Nola Fit, respectively (Table 2).
The vmax values obtained for the free and immobilized enzymes wer0e also highly comparable (Table 2). The concentrations of the active enzymes were unknown, the determination of the total protein content was assumed to potentially overestimate the concentration of the active enzyme, and therefore, the kcat values were not determined. The covalent conjugation caused a 3.9- and fourfold increase of vmax values for Saphera and Nola Fit, respectively, which may be the reason why elevated LAC hydrolysis and GOS synthesis were observed for the immobilized enzymes.
Operational Stability
Operational stabilities of CH-Saphera and CH-Nola Fit lactases were studied by determining LAC hydrolysis in homogenized pasteurized milk. While measuring enzyme activities, we considered such parameters which may be relevant for industrial applications and are determined by the milk, such as its natural pH and the concentration of LAC. In addition, measuring enzyme activity in non-diluted milk may be relevant from the viewpoint of the industrial applications, because—in contrast to buffers—the milk is a complex biological material, it contains many biomolecules which may potentially interfere with the enzymatic reactions.
We performed LAC hydrolysis by using the immobilized enzymes in 16 consecutive cycles. As it is represented in Fig. 5, repeated batch hydrolysis enabled to produce lactose-free or low-lactose milk in at least 15 effective cycles. The definition of low-lactose or lactose-free product varies by country. In Hungary, the products containing lactose in lower than 1 mg/ml concentration are defined as lactose-free, and there is no definition for low lactose (EFSA, 2010). Based on the regulation of several European countries, the low-lactose milks must contain lactose in < 10 mg/ml concentration.
Concentration of residual lactose in homogenized pasteurized milk after successive lactose hydrolysis cycles. The enzyme-coated CH beads can be used in multiple cycles for batch hydrolysis to produce lactose-free or low-lactose milk. The concentration of residual lactose (mg/ml) is plotted as a function of the number of successive lactose hydrolysis cycles in homogenized pasteurized milk, both in the case of CH-Saphera (◼) (A) and CH-Nola Fit (▲) (B) enzymes. One milligram per milliliter lactose concentration is indicated by a gray dashed line (the products containing lactose in < 1 mg/ml concentration are defined as lactose-free). All experiments were performed in triplicates; the values are presented as mean ± SD
The LAC concentration of the untreated homogenized pasteurized milk samples varied between 50 and 55 mg/ml. After batch hydrolysis, the residual LAC concentration was < 1 mg/ml in most cases and was lower than 2 mg/ml in every case (Fig. 5). Accordingly, the resulted milks were considered to be lactose-free. Our results showed no considerable loss of the enzyme activities even using the beads in multiple cycles. Both CH-Saphera and CH-Nola Fit enzymes exhibited good operational stability; their ability for sufficient in-milk hydrolysis of LAC indicates high potential for the reduction of operational costs in practical use.
The enzyme-coated beads (CH-Saphera and CH-Nola Fit) were stored at 4 °C in phosphate buffer. After storing the beads for 4 weeks, the enzymes still exhibited suitable hydrolytic activity, indicating that storage causes no enzyme inactivation. Nevertheless, a possible limitation of this study is that the effects of long-term storage on the hydrolytic and synthetic activities were not investigated in detail.
We have not observed a decrease in the size and change of the shape even using the beads in multiple consecutive cycles. The stability of the beads is provided in part by the low solubility of the chitosan at neutral and alkaline pH (Bodnár et al., 2005). Although the protonation of the amino groups improves the solubility (at acidic pH), glutaraldehyde binds to these groups during the bead activation, preventing protonation and remarkably decreasing the solubility of the polymer.
Lactose Hydrolysis and Enzymatic Synthesis of Galacto-oligosaccharides
We have tested the abilities of the CH-Saphera and CH-Nola Fit enzymes for the production of GOS-enriched milk in a process which is potentially compatible with industrial milk processing technologies. We investigated the simultaneous changes in the levels of residual LAC and produced GOSs in homogenized pasteurized milk at 37 °C, both in the case of the free and immobilized enzymes (Fig. 6). The concentration of LAC was 50–55 mg/ml prior to the treatment, which resembles the average value determined for raw milks in Hungary (Kocsis et al., 2022). The comparison of the abilities for LAC hydrolysis revealed faster depletion of LAC from the milk in the case of the immobilized enzymes, 1 h of treatment was sufficient enough to produce low-lactose ([LAC]: < 10 mg/mL) milk. Lactose-free milk ([LAC]: < 1 mg/mL) was achieved after 2 h. As compared to the immobilized ones, the free enzymes—being applied in the concentration suggested by the manufacturer—hydrolyzed the LAC more slowly and 2 h of treatment resulted in only a low-lactose but not a lactose-free milk.
Time-course of total GOSs formation and lactose (LAC) conversion in homogenized pasteurized milk using free and immobilized enzymes. The reactions were performed by using free and immobilized Saphera (A) and Nola Fit (B) enzymes at 37 °C. LAC concentration of the homogenized pasteurized milk varied between 50 and 55 mg/ml before the treatment, which was considered to be 100%. All experiments were performed in triplicates; the values are presented as mean ± SD
The abilities of the free and immobilized enzymes for transgalactosylation were also determined and compared. The untreated milk was found to be absent from GOSs. The immobilized enzymes were able to produce GOSs in a considerable amount, even in the first hour of the enzyme reaction (Fig. 6). GOSs were present in 6–8 and 8–10% of the total sugar content of the milk in the case of CH-Saphera and CH-Nola Fit enzymes, respectively. After 2 h of treatment, the GOS content of the enzyme-treated milk was considerably high in lactose-free milk. In contrast to this, the free enzymes were unable to synthesize even substantial amounts of GOS, independently from the LAC concentration (the milks that were treated with the free enzymes contained the LAC as a substrate of the transgalactosylation in higher concentration). The enzyme treatment not only decreased the lactose concentration in the milk samples but synthesis of GOSs in considerable final concentration was also achieved. The milks (or milk-derived products) that contain LAC in lower concentration (low-lactose or even lactose-free) and are enriched in GOSs may meet the needs of lactose intolerant people and those who search for dairy products containing prebiotics.
The products of transgalactosylation reaction are considered GOSs, but nowadays, in the narrow sense, only oligomeric GOSs have been defined as prebiotics (Martins et al., 2019]. Therefore, we compared not only the total GOS contents of the enzyme-treated milks (Fig. 6) but determined the level of GOS-3 (GLC-GAL-GAL) as well. The presence of GOS-3 was detected in the milk during the entire process in the case of Saphera- and Nola Fit-treated milks as well (Table 3). The highest total GOS content in the milk was higher than 9% of the total sugar content. At these GOS concentrations, the LAC concentration was even below 10%. Based on the results shown in Table 3, at > 9% GOS concentration, the LAC concentration was 2.4 mg/ml and 3.4 mg/ml for the respective CH-Saphera and CH-Nola Fit enzymes. The hydrolysis became more dominant at longer incubation times, causing a decrease in LAC and GOS concentrations as well.
After the treatment, the HPLC-MS-based analyses confirmed that the prepared milks contain tetrameric GOS-4 (GLC-GAL-GAL-GAL), indicating the presence of the prebiotic molecule at a considerable level. M/z values were calculated from the [M + Na]+ ion forms of the GOS saccharides after extraction; the same samples were injected into HPLC (Table 4).
Interestingly, the free enzymes produced only GOS-3, while both CH-Saphera and CH-Nola Fit were able to form the longer GOS-4 as well. GOS-5 was not detectable in the studied samples. These results support the importance of enzyme immobilization, because the immobilized enzymes produced GOSs in higher amount, indicating their potential for the production of GOS-enriched products. A limitation of this study is that the GOS-4 production was not quantified (Table 3), although, the total GOS content of the treated milks comprised mainly GOS-3 and GOS-4 (Table 4).
Discussion
In this study, we investigated Saphera 2600L and Nola Fit 5500 β-galactosidase enzyme solutions which have been commercialized for dairy industrial applications and contain a recombinant β-galactosidase of B. bifidum which was produced by fermentation of B. licheniformis expression strain. We aimed to study the abilities of these enzyme solutions for lactose hydrolysis and GOS synthesis, by comparing the characteristics of the free enzymes and those of immobilized CH beads. In addition, the most suitable conditions were tested for the preparation of GOS-enriched milk.
The Saphera and Nola Fit solutions used for hydrolysis and synthesis reactions were investigated by SDS-PAGE. Both were found to have high overall purity, and the pattern of the bands in the polyacrylamide gels (Fig. 1A) was in good agreement with that reported previously for Saphera 2600L (Voget et al., 2022). The MS-based protein identification revealed that the most intense bands contain the β-galactosidase of B. bifidum and are free from proteins of B. licheniformis expression strain. The manufacturers recommend to store the Saphera and Nola Fit solutions at 0–10 and 0–8 °C, respectively. Besides the recommended storage temperature (4 °C), we have tested the stabilities of the enzyme solutions as well. Based on SDS-PAGE analysis, we observed no appearance of degradation products, which implied proper stability for both enzyme solutions even after storing them for 6 months (Fig. 1). In accordance with this, the enzymes were found to retain their hydrolase activity, as well, which is important from the viewpoint of the cost-efficiency, as the solutions can be stored for months without inactivation, even at − 20 °C.
Dropwise addition is a well-known technique to form biopolymer-based beads (Flores et al., 2019; Klein et al., 2012); we also applied this method to prepare the macroparticles. The average size of the CH beads we prepared was 2 mm (Fig. 2). This is highly comparable with the mean diameters (~ 1–3 mm) of biopolymer-based beads formed from CH (Klein et al., 2016), alginate (Kurayama et al., 2020), or other polymers (Bedade et al., 2019; Erlandsson et al., 2016). The biopolymer-based macroparticles were found to be suitable for enzyme immobilization, and the importance of the particle size has also been reported (Klein et al., 2012; Biró et al., 2008). Although the nanoparticles have a high specific surface area, special techniques are required for their efficient separation. In contrast to this, the macroparticles can be separated more simply and have higher thermal stability, but a higher amount of biopolymer is necessary for the bead formation.
Amino groups of CH can be cross-linked and activated using GA to facilitate the effective covalent binding of the enzymes to the CH beads. Conditions of enzyme immobilization—including activation procedure, reaction time, or concentration of reactants—play an important role in the formation of active groups that are able to bind adequate amount of enzyme to the surface (Migneault et al., 2004). Many attempts have been made to study the enzyme immobilization especially on chitosan (Klein et al., 2016; de Albuquerque et al., 2018); moreover, some of them used commercially available enzymes (Carrara & Rubiolo, 1994; Neto et al., 2021). Nevertheless, most of the studies focused on detailed analysis of immobilization (Carrara & Rubiolo, 1994) or kinetic features (Nguyen et al., 2019; Harsa, 2014) of special reaction conditions, but the behaviors of the enzymes in milk remained to be determined. In this work, the optimal conditions of enzyme immobilization were investigated by determining the effects of GA concentration and activation time on the activities of the immobilized Nola Fit enzyme (Table 1). Both Saphera and Nola Fit B. bifidum β-galactosidases retained their activity upon immobilization. Interestingly, a relatively low (30 min) activation time was sufficient for immobilization, longer activation times (even 6 h) decreased specific activity for CH-Nola Fit in all studied GA concentrations. The application of such a short reaction time is considered to be beneficial from the viewpoint of energy- and cost-efficiency.
The quantitative limits of enzyme immobilization were also investigated by determining the efficacy of coating and different bead concentrations. The results revealed that the beads can be applied even at 50 mg/ml final concentration for sufficient activation and enzyme immobilization. Nevertheless, the immobilization was considered to be sufficient enough in the higher bead concentration range, as well, because the relative activities were between 80 and 90% if the beads were used for activation and immobilization in 75–100 bead/ml concentration (Fig. 3). Activating and coating as much beads as possible at a constant enzyme amount can decrease the costs.
Thermal stabilities of β-galactosidases may limit their application, and it is a crucial parameter of the industrial applications. The sensitivity towards temperature may depend on the type of enzyme, the support material, and the immobilization procedure itself (Urrutia et al., 2018; Flores et al., 2019). We have tested the thermal stabilities of the Saphera and Nola Fit β-galactosidases and found that the immobilized enzymes have increased thermostability as compared to the free enzymes at 50 °C (Fig. 4). A higher stability may be caused by the stabilization of the enzymes by the covalent attachment to the solid support, and due to the protective conformation that makes the immobilized enzyme more resistant to the damage of heat effect (Wahba, 2018; Zhang & Rochefort, 2011). Due to the importance of lactase’s sensitivity towards temperature in the practical applications, several immobilization studies have investigated the thermostabilities of the developed systems. In agreement with our results, it has already been reported that enzymes immobilized on solid biopolymer supports have elevated thermostability (Pan et al., 2009; Hackenhaar et al., 2021). de Albuquerque et al. reported significantly increased thermal stability for an immobilized enzyme which maintained > 60% of its initial activity after 20 h of incubation at 60 °C as compared to the free enzyme that inactivated rapidly (de Albuquerque et al., 2018). Klein et al. reported increased thermal stability of immobilized enzyme at 60 °C independently of the cross-linker as compared to the free enzyme (Klein et al., 2016). A β-galactosidase immobilized on CH macroparticles also showed increased thermal stability as compared to that immobilized on CH nanoparticles (Klein et al., 2012). In conclusion, our results represent a considerable increase in thermal stability for the immobilized Saphera and Nola Fit lactases.
We compared the effects of enzyme immobilization on the catalytic properties of Saphera and Nola Fit enzymes (Table 2). Although the values obtained for the free and the immobilized enzymes were almost identical, the immobilization caused a considerable increase of KM in the case of both enzymes. The increased KM values of the immobilized enzymes indicated decreased affinity of the immobilized enzyme to the substrate, possibly due to the conformational changes induced by covalent immobilization that may change the microenvironment of the enzyme (Wahba, 2018; Zhang & Rochefort, 2011). Another possible explanation can be the steric hindrances resulting after covalent conjugation (Gennari et al., 2018). In accordance with our results, similar effects of covalent enzyme immobilization have also been reported, the enzyme immobilization was found to increase the KM of the β-galactosidase of Aspergillus oryzae even using chitosan (free enzyme: KM = 11.5 mM, immobilized enzyme: 54.4 mM) (Flores et al., 2019) or Duolite A568 resin solid supports for immobilization (KM of the free and the immobilized enzymes was 78 ± 4 and 113 ± 6 mM, respectively) (Gürdaş et al., 2012). Despite the changes in the catalytic properties, the other experiments revealed that the enzymes retain their β-galactosidase activities upon immobilization and can be efficiently used for lactose hydrolysis.
The high operational stability of immobilized enzymes has many benefits due to multiple causes. For example, the beads can be successively re-used without any further modification or activation, and the re-use of the beads may remarkably reduce the operational costs as well. In addition, the beads (coated with the immobilized enzymes) can be easily removed from the milk via simple filtration or sedimentation techniques; consequently, the marketed product can be free from soluble enzymes unlike many lactose-free products that are supplemented with free β-galactosidase. In this work, we investigated the operational stabilities of the CH beads which were coated either with Saphera or Nola Fit enzymes; the lactase activities were measured in homogenized pasteurized milk in 16 consecutive cycles. The batch hydrolysis revealed that the enzyme-coated beads can be used efficiently in multiple cycles without the loss or remarkable decrease of their activity. Even after re-using the beads repeatedly, treatment of the milk decreased the primary 50–55 mg/ml LAC concentration, resulting in lactose-free milk (< 1 mg/ml residual LAC concentration) in most cases (Fig. 5). Many attempts have been made so far to produce excellent operational stability of immobilized enzymes through tens of cycles (Flores et al., 2019; Klein et al., 2012), but these studies were usually carried out in buffered LAC solutions and only few papers describe the LAC hydrolysis in milk (or dairy-liquid) products like buffer-diluted ultra-high temperature-treated (UHT) milk (Lima et al., 2021) or buffered milk whey permeate solution (Hackenhaar et al., 2021). We found that the operational stability of CH-Nola Fit in milk was higher than that of Nola Fit encapsulated in a sodium alginate matrix; the latter showed a dramatic decrease in its activity even after 6 cycles (Czyzewska & Trusek, 2021). Testing novel products scarcely has been performed in non-diluted and non-buffered milks; therefore, our results provide valuable information about the behavior of the immobilized enzymes in milk. Both CH-Saphera and CH-Nola Fit enzymes exhibited good operational stability; thus, their ability for sufficient in-milk LAC hydrolysis indicates high potential for the reduction of operational costs in practical application.
GOSs, as prebiotics that are functional food ingredients, have attracted the increasing attention of the researchers (Davani-Davari et al., 2019; Martins et al., 2019; Rudke et al., 2023). Several studies were performed to investigate the kinetics of LAC hydrolysis and the simultaneously occurring GOS synthesis and to determine the influencing factors of GOS production (Rodriguez-Colinas et al., 2014; Yu & O’Sullivan, 2018). It is important to note that the reaction time, the pH, the temperature, and the LAC concentration are also crucial factors that may influence the transgalactosylation reaction (Hackenhaar et al., 2021). This information is relevant in regard to the practical implementation of GOS-enriched milk production in the industry, considering the fact that the number of influencing factors to modify is limited in the industrial processes of milk production. Nevertheless, Rodriguez-Colinas et al. (2014) suggested that pasteurization should be carried out after enzymatic treatment of the milk in order to stop the reactions. It has been noted that heat treatment prior to the enzymatic process is usually preferred in the industry to avoid the Maillard reaction, which may result in milk with modified color and flavor.
In this work, we compared the abilities of the free and immobilized enzymes for LAC hydrolysis and GOS synthesis via transgalactosylation. The free enzymes were considered to be unable for the synthesis of GOS in substantial amount, while the GOSs were present in 6–8 and 8–10% of total sugar content if the milk was treated with respective CH-Saphera and CH-Nola Fit (Fig. 6). The treated milks were considered to be low-lactose while being enriched in GOSs (Table 3); mainly in GOS-3, the GOS-4 was present in a lower amount while GOS-5 was not detectable (Table 4). Unlike the free enzymes, the β-galactosidases immobilized on chitosan beads can be applied for the production of low-lactose (or even lactose-free) milk that is enriched in prebiotic GOSs.
Conclusions
The dairy industry has a trend to develop such dairy products that are fortified with molecules of beneficial features (including prebiotics) and/or have reduced sugar or fat content (Rudke et al., 2023). Studies on the enzymatic tools of potential industrial application may provide valuable information in regard to the production of dairy products with such improved features. Accordingly, in this work, we applied such methodologies for the investigation of Saphera 2600L and Nola Fit 5500 authorized lactase enzyme solutions which are considered to be easily adaptable for dairy industrial applications as described as follows.
We used a biocompatible and re-usable chitosan biopolymer matrix, which was found to be suitable for the immobilization of both Saphera and Nola Fit B. bifidum β-galactosidases; using the enzyme-coated beads of high operational stability in repeated cycles can improve cost-efficiency.
Such commercially available enzymes were used that have been authorized for the use in food industry; therefore, the food safety evaluation may be necessary only for the use of CH beads while implementing the described methods for industrial milk processing. It may be more beneficial to use authorized industrial enzymes rather than whole microbial cells because former ones contain a considerably lower amount of potential contaminants and immunogens (based on product information they are free from the microorganisms which were used for enzyme production by fermentation) (Ohmiya et al., 1977; Yu & O’Sullivan, 2018); nevertheless, there is a great potential in the application of immobilized microorganisms (de Souza et al., 2022) and whole-cell lactase catalysts (Dorau et al., 2021).
In addition, our results provide information about the abilities of Saphera 2600L and Nola Fit 5500 for LAC digestion and GOS synthesis at the level of dairy milk processing as the enzymatic reactions were carried out in the complex environment of the homogenized pasteurized milk but not buffered conditions. The enzymatic catalysis is different in milk and in buffers (Ohmiya et al., 1975); therefore, the applied industrial lactases were not further purified or supplemented with any buffer components; e.g., no cations were added, and the pH or the initial pH of the milk was not modified.
Whey is a by-product during the production of many dairy products; thus, its efficient utilization is important from the viewpoint of cost-efficiency of the dairy milk processing. Whey is a good source of milk oligosaccharides which can be concentrated, e.g., by nanofiltration (Altmann et al., 2016), and due to the abilities of β-galactosidases for the synthesis of GOSs in whey (Fischer & Kleinschmidt, 2018), they are valuable tools for the enzymatic production of various lactose-derived molecules (Nath et al., 2016) A possible limitation of our study is that the characteristics of the immobilized β-galactosidases were investigated only in homogenized pasteurized milk (skim milk); their abilities for lactose hydrolysis and GOS synthesis in other dairy liquids (e.g., dairy waste whey) remain to be tested.
The enzymes immobilized from Saphera 2600L and Nola Fit 5500 solutions exhibited elevated ability for the synthesis of GOSs; the total concentration of the synthesized GOSs was substantially higher in the milk as compared to the free β-galactosidases. In contrast to this, the free enzymes produced GOSs in remarkably lower amount and were unable for the synthesis of GOS-4. Transgalactosylation reactions from lactose may result in the synthesis of GOSs with different numbers of the comprising moieties (di-, tri-, tetra-, and pentasaccharides) as well as various configurations of the bonds (e.g., 1 → 3, 1 → 4, 1 → 6) (Nath et al., 2016). We successfully identified GOS-3 and GOS-4 synthesized by CH-Saphera and CH-Nola Fit, but the detailed investigation of the synthesized GOSs—including the determination of the bond configurations—were out of the goals and the extent of this study. Besides their ability for GOS synthesis, a beneficial property of the immobilized enzymes is that they can be easily removed from the milk; consequently, the product can be absent from a soluble lactase.
Our results provide valuable information about the features of the β-galactosidases of Saphera 2600L and Nola Fit 5500 enzyme solutions. After immobilization on the surfaces of CH beads, both enzymes can be applied for the production of such low-lactose (or lactose-free) milk which is enriched in prebiotic GOSs. The applied methodology must be tested for milk treatment on a higher scale and for determining whether it can be potentially implemented for other formulated dairy lactases.
Code Availability
Not applicable.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Altmann, K., Clawin-Rädecker, I., Hoffmann, W., & Lorenzen, P. C. (2016). Nanofiltration enrichment of milk oligosaccharides (MOS) in relation to process parameters. Food and Bioprocess Technology, 9, 1924–1936. https://doi.org/10.1007/s11947-016-1763-5
Al-Sheraji, S. H., Ismail, A., Manap, M. Y., Mustafa, S., Yusof, R. M., & Hassan, F. A. (2013). Prebiotics as functional foods: A review. Journal of Functional Foods, 5(4), 1542–1553. https://doi.org/10.1016/j.jff.2013.08.009
Anisha, G. S. (2017). β-galactosidases. Current Developments in Biotechnology and Bioengineering (pp. 395–421). Elsevier. https://doi.org/10.1016/B978-0-444-63662-1.00017-8
Annapure, U. S., & Gaur, S. S. (2022). Commercial enzymes in dairy processing. Value-Addition in Food Products and Processing Through Enzyme Technology (pp. 205–219). Elsevier. https://doi.org/10.1016/B978-0-323-89929-1.00015-9
Bedade, D. K., Sutar, Y. B., & Singhal, R. S. (2019). Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: Process parameters and removal of acrylamide from coffee. Food Chemistry, 275, 95–104. https://doi.org/10.1016/j.foodchem.2018.09.090
Biró, E., Németh, Á. S., Sisak, C., Feczkó, T., & Gyenis, J. (2008). Preparation of chitosan particles suitable for enzyme immobilization. Journal of Biochemical and Biophysical Methods, 70(6), 1240–1246. https://doi.org/10.1016/j.jprot.2007.11.005
Bodnár, M., Hartmann, J. F., & Borbély, J. (2005). Preparation and characterization of chitosan-based nanoparticles. Biomacromolecules, 6(5), 2521–2527. https://doi.org/10.1021/bm0502258
Botvynko, A., Bednářová, A., Henke, S., Shakhno, N., & Čurda, L. (2019). Production of galactooligosaccharides using various combinations of the commercial β-galactosidases. Biochemical and Biophysical Research Communications, 517(4), 762–766. https://doi.org/10.1016/j.bbrc.2019.08.001
Carrara, C. R., & Rubiolo, A. C. (1994). Immobilization of β-galactosidase on chitosan. Biotechnology Progress, 10(2), 220–224. https://doi.org/10.1021/bp00026a012
Cox, J., & Mann, M. (2008). MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature Biotechnology, 26, 1367–1372. https://doi.org/10.1038/nbt.1511
Czyzewska, K., & Trusek, A. (2021). Encapsulated NOLATM Fit 5500 lactase—An economically beneficial way to obtain lactose-free milk at low temperature. Catalysts, 11(5), 527. https://doi.org/10.3390/catal11050527
Davani-Davari, D., Negahdaripour, M., Karimzadeh, I., Seifan, M., Mohkam, M., Masoumi, S., Berenjian, A., & Ghasemi, Y. (2019). Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods, 8(3), 92. https://doi.org/10.3390/foods8030092
de Albuquerque, T. L., Gomes, S. D. L., D’Almeida, A. P., Fernandez-Lafuente, R., Gonçalves, L. R. B., & Rocha, M. V. P. (2018). Immobilization of β-galactosidase in glutaraldehyde-chitosan and its application to the synthesis of lactulose using cheese whey as feedstock. Process Biochemistry, 73, 65–73. https://doi.org/10.1016/j.procbio.2018.08.010
de Souza, W. F. C., Almeida, F. L. C., de Melo, A. M., Soares, A. S. P., Forte, M. B. S., de Castro, R. J. S., & Sato, H. H. (2022). Immobilization techniques on bioprocesses: Current applications regarding enzymes, microorganisms, and essential oils. Food and Bioprocess Technology, 15, 1449–1476. https://doi.org/10.1007/s11947-022-02780-w
Dekker, P. J. T., Koenders, D., & Bruins, M. J. (2019). Lactose-free dairy products: Market developments, production, nutrition and health benefits. Nutrients, 11(3), 551. https://doi.org/10.3390/nu11030551
Deng, Y., Misselwitz, B., Dai, N., & Fox, M. (2015). Lactose intolerance in adults: Biological mechanism and dietary management. Nutrients, 7(9), 8020–8035. https://doi.org/10.3390/nu7095380
Dominguez, A. L., Rodrigues, L. R., Lima, N. M., & Teixeira, A. (2014). An overview of the recent developments on Fructooligosaccharide production and applications. Food and Bioprocess Technology, 7, 324–337. https://doi.org/10.1007/s11947-013-1221-6
Dorau, R., Jensen, P. R., & Solem, C. (2021). Purified lactases versus whole-cell lactases-the winner takes it all. Applied Microbiology and Biotechnology, 105(12), 4943–4955. https://doi.org/10.1007/s00253-021-11388-7
EFSA. (2010). EFSA panel on dietetic products, nutrition and allergies (NDA); scientific opinion on lactose thresholds in lactose intolerance and galactosaemia. EFSA Journal, 8(9):1777. https://doi.org/10.2903/j.efsa.2010.1777
Erlandsson, J., López Durán, V., Granberg, H., Sandberg, M., Larsson, P. A., & Wågberg, L. (2016). Macro- and mesoporous nanocellulose beads for use in energy storage devices. Applied Materials Today, 5, 246–254. https://doi.org/10.1016/j.apmt.2016.09.008
Fischer, C., & Kleinschmidt, T. (2018). Synthesis of galactooligosaccharides in milk and whey: A review. Comprehensive Reviews in Food Science and Food Safety, 17(3), 678–697. https://doi.org/10.1111/1541-4337.12344
Flores, E. E. E., Cardoso, F. D., Siqueira, L. B., Ricardi, N. C., Costa, T. H., Rodrigues, R. C., Klein, M. P., & Hertz, P. F. (2019). Influence of reaction parameters in the polymerization between genipin and chitosan for enzyme immobilization. Process Biochemistry, 84, 73–80. https://doi.org/10.1016/j.procbio.2019.06.001
Füreder, V., Rodriguez-Colinas, B., Cervantes, F. V., Fernandez-Arrojo, L., Poveda, A., Jimenez-Barbero, J., Ballesteros, A. O., & Plou, F. J. (2020). Selective synthesis of galactooligosaccharides containing β(1→3) linkages with β-galactosidase from Bifidobacterium bifidum (Saphera). Journal of Agricultural and Food Chemistry, 68(17), 4930–4938. https://doi.org/10.1021/acs.jafc.0c00997
Gennari, A., Mobayed, F. H., Volpato, G., & de Souza, C. F. V. (2018). Chelation by collagen in the immobilization of Aspergillus oryzae β-galactosidase: A potential biocatalyst to hydrolyze lactose by batch processes. International Journal of Biological Macromolecules, 109, 303–310. https://doi.org/10.1016/j.ijbiomac.2017.12.088
González-Delgado, I., Segura, Y., Martín, A., López-Muñoz, M.-J., & Morales, G. (2018). β-galactosidase covalent immobilization over large-pore mesoporous silica supports for the production of high galacto-oligosaccharides (GOS). Microporous and Mesoporous Materials, 257, 51–61. https://doi.org/10.1016/j.micromeso.2017.08.020
Gosling, A., Stevens, G. W., Barber, A. R., Kentish, S. E., & Gras, S. L. (2010). Recent advances refining galactooligosaccharide production from lactose. Food Chemistry, 121, 307–318. https://doi.org/10.1016/j.foodchem.2009.12.063
Goulas, A., Tzortzis, G., & Gibson, G. R. (2007). Development of a process for the production and purification of α- and β-galactooligosaccharides from Bifidobacterium bifidum NCIMB 41171. International Dairy Journal, 17, 648–656. https://doi.org/10.1016/j.idairyj.2006.08.010
Grosová, Z., Rosenberg, M., & Rebroš, M. (2008). Perspectives and applications of immobilised β-galactosidase in food industry – A review. Czech Journal of Food Sciences, 26(No. 1), 1–14. https://doi.org/10.17221/1134-CJFS
Gürdaş, S., Güleç, H. A., & Mutlu, M. (2012). Immobilization of Aspergillus oryzae β-galactosidase onto Duolite A568 resin via simple adsorption mechanism. Food and Bioprocess Technology, 5, 904–911. https://doi.org/10.1007/s11947-010-0384-7
Hackenhaar, C. R., Spolidoro, L. S., Flores, E. E. E., Klein, M. P., & Hertz, P. F. (2021). Batch synthesis of galactooligosaccharides from co-products of milk processing using immobilized β-galactosidase from Bacillus circulans. Biocatalysis and Agricultural Biotechnology, 36, 102136. https://doi.org/10.1016/j.bcab.2021.102136
Hajdu, I., Bodnár, M., Filipcsei, G., Hartmann, J. F., Daróczi, L., Zrínyi, M., & Borbély, J. (2008). Nanoparticles prepared by self-assembly of chitosan and poly-γ-glutamic acid. Colloid and Polymer Science, 286(3), 343–350. https://doi.org/10.1007/s00396-007-1785-7
Harsa, S. T. (2014). β-galactosidase immobilization on chitosan-hydroxyapatite complex: Effects of immobilization conditions. Journal of Nutritional Health & Food Engineering, 1(1). https://doi.org/10.15406/jnhfe.2014.01.00004
Hidalgo-Cantabrana, C., Delgado, S., Ruiz, L., Ruas-Madiedo, P., Sánchez, B., & Margolles, A. (2017). Bifidobacteria and their health-promoting effects. Microbiology spectrum, 5(3), https://doi.org/10.1128/microbiolspec.BAD-0010-2016. https://doi.org/10.1128/microbiolspec.BAD-0010-2016
Husain, Q., Ansari, S. A., Alam, F., & Azam, A. (2011). Immobilization of Aspergillus oryzae β galactosidase on zinc oxide nanoparticles via simple adsorption mechanism. International Journal of Biological Macromolecules, 49(1), 37–43. https://doi.org/10.1016/j.ijbiomac.2011.03.011
Iqbal, S., Nguyen, T.-H., Nguyen, T. T., Maischberger, T., & Haltrich, D. (2010). β-Galactosidase from Lactobacillus plantarum WCFS1: Biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydrate Research, 345(10), 1408–1416. https://doi.org/10.1016/j.carres.2010.03.028
Khan, M., Husain, Q., & Ahmad, N. (2019). Elucidating the binding efficacy of β-galactosidase on polyaniline-chitosan nanocomposite and polyaniline-chitosan-silver nanocomposite: Activity and molecular docking insights: Binding efficacy of β-galactosidase on PANI-CS-NC and PANI-CS-Ag NC. Journal of Chemical Technology & Biotechnology, 94(3), 837–849. https://doi.org/10.1002/jctb.5831
Klein, M. P., Hackenhaar, C. R., Lorenzoni, A. S. G., Rodrigues, R. C., Costa, T. M. H., Ninow, J. L., & Hertz, P. F. (2016). Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and β-d-galactosidase immobilization. Carbohydrate Polymers, 137, 184–190. https://doi.org/10.1016/j.carbpol.2015.10.069
Klein, M. P., Nunes, M. R., Rodrigues, R. C., Benvenutti, E. V., Costa, T. M. H., Hertz, P. F., & Ninow, J. L. (2012). Effect of the support size on the properties of β-galactosidase immobilized on chitosan: Advantages and disadvantages of macro and nanoparticles. Biomacromolecules, 13(8), 2456–2464. https://doi.org/10.1021/bm3006984
Kocabaş, D. S., Lyne, J., & Ustunol, Z. (2022). Hydrolytic enzymes in the dairy industry: Applications, market and future perspectives. Trends in Food Science & Technology, 119, 467–475. https://doi.org/10.1016/j.tifs.2021.12.013
Kocsis, R., Süle, J., Nagy, P., Gál, J., Tardy, E., Császár, G., & Rácz, B. (2022). Annual and seasonal trends in cow’s milk quality determined by FT-MIR spectroscopy in Hungary between 2011 and 2020. Acta Veterinaria Hungarica. https://doi.org/10.1556/004.2022.00019
Kurayama, F., Mohammed Bahadur, N., Furusawa, T., Sato, M., & Suzuki, N. (2020). Facile preparation of aminosilane-alginate hybrid beads for enzyme immobilization: Kinetics and equilibrium studies. International Journal of Biological Macromolecules, 150, 1203–1212. https://doi.org/10.1016/j.ijbiomac.2019.10.130
Lima, P. C., Gazoni, I., de Carvalho, A. M. G., Bresolin, D., Cavalheiro, D., de Oliveira, D., & Rigo, E. (2021). β-galactosidase from Kluyveromyces lactis in genipin-activated chitosan: An investigation on immobilization, stability, and application in diluted UHT milk. Food Chemistry, 349, 129050. https://doi.org/10.1016/j.foodchem.2021.129050
Lomer, M. C. E., Parkes, G. C., & Sanderson, J. D. (2007). Review article: Lactose intolerance in clinical practice - Myths and realities: Review: Lactose intolerance in clinical practice. Alimentary Pharmacology & Therapeutics, 27(2), 93–103. https://doi.org/10.1111/j.1365-2036.2007.03557.x
Majore, K. & Ciproviča,I. (2020). Optimisation of lactose hydrolysis by combining solids and ß-galactosidase concentrations in whey permeates. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences, 74(4) 263–269. https://doi.org/10.2478/prolas-2020-0041
Mano, M. C. R., Paulino, B. N., & Pastore, G. M. (2019). Whey permeate as the raw material in galacto-oligosaccharide synthesis using commercial enzymes. Food Research International, 124, 78–85. https://doi.org/10.1016/j.foodres.2018.09.019
Manzi, P., & Pizzoferrato, L. (2013). HPLC determination of lactulose in heat treated milk. Food and Bioprocess Technology, 6(3), 851–857. https://doi.org/10.1007/s11947-011-0700-x
Martins, G. N., Ureta, M. M., Tymczyszyn, E. E., Castilho, P. C., & Gomez-Zavaglia, A. (2019). Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Frontiers in Nutrition, 6, 78. https://doi.org/10.3389/fnut.2019.00078
Migneault, I., Dartiguenave, C., Bertrand, M. J., & Waldron, K. C. (2004). Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques, 37(5), 790–802. https://doi.org/10.2144/04375RV01
Mohammadi, A., Jafari, S. M., Mahoonak, A. S., & Ghorbani, M. (2021). Liposomal/nanoliposomal encapsulation of food-relevant enzymes and their application in the food industry. Food and Bioprocess Technology, 14, 23–38. https://doi.org/10.1007/s11947-020-02513-x
Nath, A., Verasztó, B., Basak, S., Koris, A., Kovács, Z., & Vatai, Gy. (2016). Synthesis of lactose-derived nutraceuticals from dairy waste whey-A review. Food and Bioprocess Technology, 9, 16–48. https://doi.org/10.1007/s11947-015-1572-2
Neto, C. A. C. G., Silva, N. C. G., & e, de Oliveira Costa, T., de Albuquerque, T. L., Gonçalves, L. R. B., Fernandez-Lafuente, R., & Rocha, M. V. P. (2021). The β-galactosidase immobilization protocol determines its performance as catalysts in the kinetically controlled synthesis of lactulose. International Journal of Biological Macromolecules, 176, 468–478. https://doi.org/10.1016/j.ijbiomac.2021.02.078
Nguyen, V. D., Styevkó, G., Madaras, E., Haktanirlar, G., Tran, A. T. M., Bujna, E., Dam, M. S., & Nguyen, Q. D. (2019). Immobilization of β-galactosidase on chitosan-coated magnetic nanoparticles and its application for synthesis of lactulose-based galactooligosaccharides. Process Biochemistry, 84, 30–38. https://doi.org/10.1016/j.procbio.2019.05.021
Nijpels, H. H. (1981). Lactases and their applications. In G. G. Birch, N. Blakebrough, & K. J. Parker (Eds.), Enzymes and Food Processing (pp. 89–104). Dordrecht: Springer, Netherlands. https://doi.org/10.1007/978-94-011-6740-6_6
O’Callaghan, A., & van Sinderen, D. (2016). Bifidobacteria and their role as members of the human gut microbiota. Frontiers in Microbiology, 7, 925. https://doi.org/10.3389/fmicb.2016.00925
Ohmiya, K., Ohashi, H., Kobayashi, T., & Shimizu, S. (1977). Hydrolysis of lactose by immobilized microorganisms. Applied and Environmental Microbiology, 33(1), 137–146. https://doi.org/10.1128/aem.33.1.137-146.1977
Ohmiya, K., Terao, C., Shimizu, S., & Kobayashi, T. (1975). Immobilization of β-galactosidase by polyacrylamide in the presence of protective agents. Agricultural and Biological Chemistry, 39(2), 491–498. https://doi.org/10.1080/00021369.1975.10861617
Otieno, D. O. (2010). Synthesis of β-galactooligosaccharides from lactose using microbial β-galactosidases. Comprehensive Reviews in Food Science and Food Safety, 9(5), 471–482. https://doi.org/10.1111/j.1541-4337.2010.00121.x
Pan, C., Hu, B., Li, W., Sun, Y., Ye, H., & Zeng, X. (2009). Novel and efficient method for immobilization and stabilization of β-d-galactosidase by covalent attachment onto magnetic Fe3O4–chitosan nanoparticles. Journal of Molecular Catalysis b: Enzymatic, 61(3–4), 208–215. https://doi.org/10.1016/j.molcatb.2009.07.003
Panesar, P. S., Kumari, S., & Panesar, R. (2010). Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Research, 2010, 473137. https://doi.org/10.4061/2010/473137
Panesar, P. S., Panesar, R., Singh, R. S., Kennedy, J. F., & Kumar, H. (2006). Microbial production, immobilization and applications of β-D-galactosidase. Journal of Chemical Technology & Biotechnology, 81(4), 530–543. https://doi.org/10.1002/jctb.1453
Polyák, A., Hajdu, I., Bodnár, M., Dabasi, G., Jóba, R. P., Borbély, J., & Balogh, L. (2014). Folate receptor targeted self-assembled chitosan-based nanoparticles for SPECT/CT imaging: Demonstrating a preclinical proof of concept. International Journal of Pharmaceutics, 474(1–2), 91–94. https://doi.org/10.1016/j.ijpharm.2014.07.055
Popescu, L., Bulgaru, V., & Siminiuc, R. (2021). Effect of temperature, pH and amount of enzyme used in the lactose hydrolysis of milk. Food and Nutrition Sciences, 12, 1243–1254. https://doi.org/10.4236/fns.2021.1212091
Ratajczak, A. E., Rychter, A. M., Zawada, A., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). Lactose intolerance in patients with inflammatory bowel diseases and dietary management in prevention of osteoporosis. Nutrition, 82, 111043.
Roberfroid, M., Gibson, G. R., Hoyles, L., McCartney, A. L., Rastall, R., Rowland, I., Wolvers, D., Watzl, B., Szajewska, H., Stahl, B., Guarner, F., Respondek, F., Whelan, K., Coxam, V., Davicco, M. J., Léotoing, L., Wittrant, Y., Delzenne, N. M., Cani, P. D., … Meheust, A. (2010). Prebiotic effects: Metabolic and health benefits. The British Journal of Nutrition, 104(Suppl 2), S1–S63. https://doi.org/10.1017/S0007114510003363
Rodriguez-Colinas, B., Fernandez-Arrojo, L., Ballesteros, A. O., & Plou, F. J. (2014). Galactooligosaccharides formation during enzymatic hydrolysis of lactose: Towards a prebiotic-enriched milk. Food Chemistry, 145, 388–394. https://doi.org/10.1016/j.foodchem.2013.08.060
Rudke, C. R. M., Camelo-Silva, C., Rudke, A. R., Prudencio, E. S., & de Andrade, C. J. (2023). Trends in dairy products: New ingredients and ultrasound-based processing. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-023-03153-7
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R., & Rastall, R. A. (2019). Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nature Reviews Gastroenterology & Hepatology, 16(10), 605–616. https://doi.org/10.1038/s41575-019-0173-3
Sangwan, V., Tomar, S. K., Ali, B., Singh, R. R. B., & Singh, A. K. (2015). Galactooligosaccharides reduce infection caused by Listeria monocytogenes and modulate IgG and IgA levels in mice. International Dairy Journal, 41, 58–63. https://doi.org/10.1016/j.idairyj.2014.09.010
Schmidt, C. M., Balinger, F., Conrad, J., Günther, J., Beifuss, U., & Hinrichs, J. (2020). Enzymatic generation of lactulose in sweet and acid whey: Optimization of feed composition and structural elucidation of 1-lactulose. Food Chemistry, 305, 125481. https://doi.org/10.1016/j.foodchem.2019.125481
Sharp, E., D’Cunha, N. M., Ranadheera, C. S., Vasiljevic, T., Panagiotakos, D. B., & Naumovski, N. (2021). Effects of lactose-free and low-lactose dairy on symptoms of gastrointestinal health: A systematic review. International Dairy Journal, 114, 104936. https://doi.org/10.1016/j.idairyj.2020.104936
Souza, A. F. C. E., Gabardo, S., & Coelho, R. J. S. (2022). Galactooligosaccharides: Physiological benefits, production strategies, and industrial application. Journal of Biotechnology, 359, 116–129. https://doi.org/10.1016/j.jbiotec.2022.09.020
Storhaug, C. L., Fosse, S. K., & Fadnes, L. T. (2017). Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology, 2(10), 738–746. https://doi.org/10.1016/S2468-1253(17)30154-1
Urrutia, P., Bernal, C., Wilson, L., & Illanes, A. (2018). Use of chitosan heterofunctionality for enzyme immobilization: β-galactosidase immobilization for galacto-oligosaccharide synthesis. International Journal of Biological Macromolecules, 116, 182–193. https://doi.org/10.1016/j.ijbiomac.2018.04.112
Urrutia, P., Rodriguez-Colinas, B., Fernandez-Arrojo, L., Ballesteros, A. O., Wilson, L., Illanes, A., & Plou, F. J. (2013). Detailed analysis of galactooligosaccharides synthesis with β-galactosidase from Aspergillus oryzae. Journal of Agricultural and Food Chemistry, 61(5), 1081–1087. https://doi.org/10.1021/jf304354u
Vera, C., Guerrero, C., Aburto, C., Cordova, A., & Illanes, A. (2020). Conventional and non-conventional applications of β-galactosidases. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1868(1), 140271. https://doi.org/10.1016/j.bbapap.2019.140271
Voget, C., Borrajo, A., Pedrazzi, C. (2022) Lactose hydrolysis in milk using a commercial recombinant β-galactosidase (lactase) from Bifidobacterium bifidum. Food Science and Technology, 42. https://doi.org/10.1590/fst.27622.
Wahba, M. I. (2018). Processed gellan gum beads as covalent immobilization carriers. Biocatalysis and Agricultural Biotechnology, 14, 270–278. https://doi.org/10.1016/j.bcab.2018.03.019
Walker, J. M. (1996). SDS polyacrylamide gel electrophoresis of proteins. In J. M. Walker (Ed.), The Protein Protocols Handbook. Humana Press: Springer Protocols Handbooks.
Wolf, M., Belfiore, L. A., Tambourgi, E. B., & Paulino, A. T. (2019). Production of low-dosage lactose milk using lactase immobilised in hydrogel. International Dairy Journal, 92, 77–83. https://doi.org/10.1016/j.idairyj.2018.12.004
Wolf, M., Tambourgi, E. B., & Paulino, A. T. (2021). Stability of β-D-galactosidase immobilized in polysaccharide-based hydrogels. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 609, 125679. https://doi.org/10.1016/j.colsurfa.2020.125679
Yu, L., & O’Sullivan, D. J. (2018). Immobilization of whole cells of Lactococcus lactis containing high levels of a hyperthermostable β-galactosidase enzyme in chitosan beads for efficient galacto-oligosaccharide production. Journal of Dairy Science, 101(4), 2974–2983. https://doi.org/10.3168/jds.2017-13770
Zhang, Y., & Rochefort, D. (2011). Activity, conformation and thermal stability of laccase and glucose oxidase in poly(ethyleneimine) microcapsules for immobilization in paper. Process Biochemistry, 46(4), 993–1000. https://doi.org/10.1016/j.procbio.2011.01.006
Zhang, W., Jia, Q., Han, M., Zhang, X., Guo, L., Sun, S., Yin, W., Bo, C., Han, R., & Sai, L. (2023). Bifidobacteria in disease: From head to toe. Folia Microbiologica. https://doi.org/10.1007/s12223-023-01087-3.Advanceonlinepublication.10.1007/s12223-023-01087-3
Acknowledgements
The authors are grateful to the members of the Laboratory of Retroviral Biochemistry research group and those of the Proteomics Core Facility at the Department of Biochemistry and Molecular Biology (University of Debrecen). The authors extend their gratitude to Zsolt Sarang for critically reading the manuscript before submission (Department of Biochemistry and Molecular Biology, University of Debrecen).
Funding
Open access funding provided by University of Debrecen. This work was supported by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the 2019-1.1.1-PIACI-KFI funding scheme (Project no. 2019-1.1.1-PIACI-KFI-2019-00109). This paper was also supported by Grant No. FK-132385 (to T.N.) from National Research, Development and Innovation Office (NKFI) and by the GINOP-2.3.3-15-2016-00020 project. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
Conceptualization, Magdolna Bodnár, Erika Fazekas, Tibor Nagy, Krisztina Kerekes, Eszter Prépost, János András Mótyán; methodology, Magdolna Bodnár, Erika Fazekas, Tibor Nagy, Noémi Miltner, Gergő Kalló, János András Mótyán, Krisztina Kerekes, Eszter Prépost, János András Mótyán; validation, Magdolna Bodnár, Erika Fazekas, Tibor Nagy, Gergő Kalló, Krisztina Kerekes, Eszter Prépost, János András Mótyán; formal analysis, Magdolna Bodnár, Erika Fazekas, Tibor Nagy, Noémi Miltner, Gergő Kalló, Krisztina Kerekes, János András Mótyán; investigation, Magdolna Bodnár, Erika Fazekas, Tibor Nagy, Noémi Miltner, Gergő Kalló, Krisztina Kerekes, János András Mótyán; resources, Eszter Prépost; data curation, Magdolna Bodnár, Erika Fazekas, Gergő Kalló, Tibor Nagy, Krisztina Kerekes, János András Mótyán; writing original draft preparation, Magdolna Bodnár, Erika Fazekas, Tibor Nagy, Noémi Miltner, Gergő Kalló, János András Mótyán, Krisztina Kerekes, Eszter Prépost; writing review and editing, Magdolna Bodnár, János András Mótyán; visualization, Magdolna Bodnár, Erika Fazekas, Noémi Miltner, Gergő Kalló, Krisztina Kerekes, János András Mótyán; funding acquisition, Eszter Prépost, Tibor Nagy. All authors agreed the submission of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bodnár, M., Fazekas, E., Nagy, T. et al. Synthesis of Galacto-oligosaccharides in Milk by Using Bifidobacterium bifidum β-galactosidases (Saphera 2600L and Nola Fit 5500) Immobilized on Chitosan Beads. Food Bioprocess Technol 17, 1–20 (2024). https://doi.org/10.1007/s11947-023-03222-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03222-x