Abstract
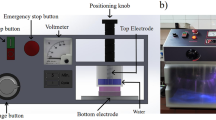
Peanut protein isolate (PPI) solutions were modified by dielectric barrier discharge (DBD) cold plasma (CP) treatment. Effects of CP treatment on the solubility, emulsion stability, and water holding capacity (WHC) of peanut protein were studied. The results showed a significant improvement in solubility, emulsion stability, and WHC following CP treatment. CP treatment resulted in the unfolding of PPI structure, thereby increasing the β-sheet and random coil content and decreasing the α-helix and β-turn content, as analyzed by Fourier-transform infrared spectroscopy. Low-field nuclear magnetic resonance showed an increase in the peak area of T 21 relaxation time by CP treatment and the change in T 21 peak area was in agreement with the result of WHC. This study demonstrated that CP may be successfully applied as a method to modify the functionality of PPI.







Similar content being viewed by others
References
Attri, P., et al. (2013). Utility of plasma: a new road from physics to chemistry. RSC Advances, 3(31), 12540–12567.
Bertram, H. C., et al. (2002). Continuous distribution analysis of T2, relaxation in meat—an approach in the determination of water-holding capacity. Meat Science, 60(3), 279.
Beveridge, T., et al. (1974). Determination of SH- and SS-groups in some food proteins using Ellman’s reagent. Journal of Food Science, 39(1), 49–51.
Bianchi-Hall, C. M., et al. (1993). Diversity of seed storage protein patterns in wild peanut (Arachis, Fabaceae) species. Plant Systematics and Evolution, 186(1–2), 1–15.
Boye, J. I., Alli, I., & Ismail, A. A. (1996). Interactions involved in the gelation of bovine serum albumin. Journal of Agricultural and Food Chemistry., 44(4), 996–1004.
Caessens, P. W., et al. (1999). The adsorption-induced secondary structure of beta-casein and of distinct parts of its sequence in relation to foam and emulsion properties. Biochimica et Biophysica Acta, 1430(1), 73.
Cataldo, F. (2003). On the action of ozone on proteins. Polymer Degradation & Stability, 82(1), 105–114.
Daussant, J., et al. (1969). Immunochemical studies on arachis hypogaea proteins with particular reference to the reserve proteins. I. Characterization, distribution, and properties of α-arachin and α-conarachin. Plant Physiology, 44(4), 471.
Davies, M. J. (2005). The oxidative environment and protein damage. Biochimica et Biophysica Acta, 1703, 93.
Dong, S., et al. (2017). Effects of dielectric barrier discharges (DBD) cold plasma treatment on physicochemical and structural properties of zein powders. Food & Bioprocess Technology, 10(3), 434–444.
Donovan, J. W., & Beardslee, R. A. (1975). Heat stabilization produced by protein-protein association. A differential scanning calorimetric study of the heat denaturation of the trypsin-soybean trypsin inhibitor and trypsin-ovomucoid complexes. Journal of Biological Chemistry, 250(6).
Durand, A., et al. (2003). Particle sizes and stability of the bovine, cereal and grain milks. Food Hydrocolloids, 17(5), 671–678.
Eaton, P. (2006). Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radical Biology and Medicine, 40(11), 1889–1899.
Ellman, G. D. (1959). Tissue sulfhydryl groups. Archives Biochemistry Biophysics, 82, 70–72.
Gianferri, R., et al. (2007). A low-resolution and high-resolution nuclear magnetic resonance integrated approach to investigate the physical structure and metabolic profile of Mozzarella di Bufala Campana cheese. International Dairy Journal, 17, 167–176.
Haris, P. I., & Severcan, F. (1999). FTIR spectroscopic characterization of protein structure in aqueous and non-aqueous media. Journal of Molecular Catalysis B Enzymatic, 7, 207–221.
Haskard, C. A., & Li-Chan, E. C. Y. (1998). Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANSÀ) fluorescent probes. J. Agric Food Chemistry, 46, 2671–2677.
He, X. H., et al. (2014). Effects of high pressure on the physicochemical and functional properties of peanut protein isolates. Food Hydrocolloids, 36(5), 123–129.
Hoying, L., et al. (2008). Study of conformation of vicilin from Dolichos lablab and Phaseolus calcaratus by Fourier-transform infrared spectroscopy and differential scanning calorimetry. Food Research Internationa, 41(7), 720–729.
J. M. Berg, J. L. Tymoczko and L. (2001). Stryer, Biochemistry, W. H. Freeman and Company. New York, p. 84.
Kim, J. E., et al. (2014). Microbial decontamination of red pepper powder by cold plasma. Food Microbiology, 38, 128.
Kim, S., et al. (2004). Characterization of zein modified with a mild cross-linking agent. Industrial Crops and Products, 20(3), 291–300.
Kocher, P. N., & Foegeding, E. A. (1993). Microcentrifuge-based method for measuring water-holding of protein gels. Journal of Food Science, 58, 1040–1046.
Kong, B., & Xiong, Y. L. (2006). Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. Journal of Agricultural and Food Chemistry, 54(16), 6059–6068.
Kurganov, B. I., et al. (1997). Analysis of differential scanning calorimetry data for proteins. Criteria of validity of one-step mechanism of irreversible protein denaturation. Biophysical Chemistry, 69(2–3), 125–135.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Li, T., et al. (2014). Water distribution in tofu and application of T2 relaxation measurements in determination of tofu’s water-holding capacity. Journal of Agricultural & Food Chemistry, 62(8), 594–601.
Lu, M., et al. (2002). Differential scanning calorimetric and circular dichroistic studies on plant antifreeze proteins. Journal of Thermal Analysis and Calorimetry, 67(3), 689–698.
Ma, T., et al. (2017). Influence of extraction and solubilizing treatments on the molecular structure and functional properties of peanut protein. LWT - Food Science and Technology, 79, 197–204.
Wiśniewska, M., et al. (2013). Effect of solution pH on the stability of mixed silica-alumina suspension in the presence of polyacrylic acid (PAA) with different molecular weights. Central European Journal of Chemistry, 11, 101–110.
Molina, E., et al. (2002). Soy protein pressure-induced gels. Food Hydrocolloids 16:625–632.
Monteiro, et al. (1994). Effect of proteases on arachin, conarachin I, and conarachin II from peanut (Arachis hypogaea L.) Journal of Agricultural and Food Chemistry, 42, 268–273.
Ochoa-Rivas, A., et al. (2016). Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: effects in yield and functional properties of protein isolates. Food & Bioprocess Technology, 1–13.
Mu, L., et al. (2010). Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. Journal of Agricultural & Food Chemistry, 58(7), 4494.
Oehmigen, K., et al. (2010). The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes and Polymers, 7(3–4), 250–257.
Offer, G., & Knight, P. (1988). Structural basis of water-holding in meat. 1. General principles and water uptake in meat processing. Developments in Meat Science.
Raemy, A. (2003). Behavior of foods studied by thermal analysis: introduction. Journal of Thermal Analysis and Calorimetry, 71(1), 273–278.
Relkin, P. (1994). Differential scanning calorimetry: a useful tool for studying protein denaturation. Thermochimica Acta, 246(2), 371–386.
Segat, A., et al. (2014). Effects of ozone processing on chemical, structural and functional properties of whey protein isolate. Food Research International, 66, 365–372.
Segat, A., et al. (2015). Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innovative Food Science & Emerging Technologies, 29, 247–254.
Thorarinsdottir, K. A., et al. (2002). Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chemistry, 77, 377–385.
Traylor, M. J., et al. (2011). Long-term antibacterial efficacy of air plasma-activated water. Journal of Physics D: Applied Physics, 44(47), 472001.
Wang, S. F., and Smith, D. M. (1994). Poultry muscle proteins and heat-induced gelation. Poultry Science Reviews.
Wang, Z., et al. (2014). Relationship between secondary structure and surface hydrophobicity of soybean protein isolate subjected to heat treatment. Journal of Chemistry, 2014(5), 1–10.
Wisniewska, M. (2010). Influences of polyacrylic acid adsorption and temperature on the alumina suspension stability. Powder Technology, 198(2), 258–266.
Wu, H., et al. (2009). Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Research International, 42(3), 343–348.
Yamada, T., et al. (1979). Isolation and some properties of arachin subunits. Agricultural and Biological Chemistry, 43, 2563.
Yang, H., et al. (2015). Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nature Protocols, 10, 382–396.
Yu, J. M., et al. (2007). Peanut protein concentrate: production and functional properties as affected by processing. Food Chemistry, 103(1), 121–129.
Zhang, Q. T., et al. (2014). Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food & Bioproducts Processing, 92(1), 30–37.
Zhao, G. L., et al. (2011). Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chemistry, 127(4), 1438–1443.
Funding
This research was financially supported by the National Science Foundation of China (Grant Nos. 31271974 and 31501503) and Tianjin Food Safety and Low Carbon Manufacturing Collaborative Innovation Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, H., Dong, S., Han, F. et al. Effects of Dielectric Barrier Discharge (DBD) Cold Plasma Treatment on Physicochemical and Functional Properties of Peanut Protein. Food Bioprocess Technol 11, 344–354 (2018). https://doi.org/10.1007/s11947-017-2015-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-2015-z