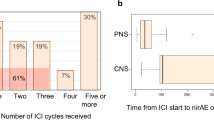
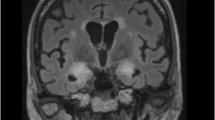
Abstract
Purpose of Review
Immune checkpoint inhibition for treatment of metastatic cancers is recognized to cause diverse immune-mediated neurological syndromes. This review will discuss current knowledge about the frequency and varied presentation of these syndromes affecting the peripheral nervous system as well as detail important diagnostic and management considerations.
Recent Findings
Immune-related adverse events affect the peripheral nervous system more often than the central nervous system, and rates are likely underestimated. Most data regarding neurological immune–related adverse events are retrospective, and prospective studies are needed. These immune-mediated peripheral nervous system syndromes can be severe and contribute to mortality. Discontinuation of ICI therapy combined with aggressive medical management can improve outcomes. Data to inform evidence-based treatment approaches, particularly in moderate to severe events, are needed.
Summary
It is important to recognize the association between immune-mediated peripheral nervous system syndromes and immune checkpoint therapy. These syndromes can be phenotypically diverse, but conditions such as acquired demyelinating neuropathy, myositis, and myasthenia gravis predominate. It is important for neurologists to recognize and promptly diagnose these conditions and manage these patients in a multidisciplinary setting to improve outcomes.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–8.
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. https://doi.org/10.1016/j.intimp.2018.06.001.
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. https://doi.org/10.1056/NEJMoa1003466.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. https://doi.org/10.1056/NEJMoa1200690.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. https://doi.org/10.1056/NEJMoa1200694.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. https://doi.org/10.1097/COC.0000000000000239.
Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin Proc. 2019;94(7):1321–9. https://doi.org/10.1016/j.mayocp.2019.03.012.
Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519. https://doi.org/10.1001/jamaoncol.2019.5570.
Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. https://doi.org/10.1186/s40425-019-0805-8.
Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer. 2019;19(1):974. https://doi.org/10.1186/s12885-019-6150-y.
Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung Cancer. Clin Lung Cancer. 2019;20(3):201–7. https://doi.org/10.1016/j.cllc.2018.10.002.
Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–85. https://doi.org/10.1007/s00432-018-2805-3.
Kao JC, Liao B, Markovic SN, Klein CJ, Naddaf E, Staff NP, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017;74(10):1216–22. https://doi.org/10.1001/jamaneurol.2017.1912.
Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–25. https://doi.org/10.1016/j.ejca.2016.02.024.
Bruna J, Argyriou AA, Anastopoulou GG, Alemany M, Nadal E, Kalofonou F, et al. Incidence and characteristics of neurotoxicity in immune checkpoint inhibitors with focus on neuromuscular events: experience beyond the clinical trials. J Peripher Nerv Syst. 2020;25:171–7. https://doi.org/10.1111/jns.12371.
Tison A, Quere G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a Nationwide, Multicenter Cohort Study. Arthritis Rheum. 2019;71(12):2100–11. https://doi.org/10.1002/art.41068.
Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. 2017;73:1–8. https://doi.org/10.1016/j.ejca.2016.12.001.
Mohn N, Beutel G, Gutzmer R, Ivanyi P, Satzger I, Skripuletz T. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy-review of the literature and future outlook. J Clin Med. 2019;8(11). https://doi.org/10.3390/jcm8111777.
Spain L, Walls G, Julve M, O'Meara K, Schmid T, Kalaitzaki E, et al. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol. 2017;28(2):377–85. https://doi.org/10.1093/annonc/mdw558.
Puwanant A, Isfort M, Lacomis D, Zivkovic SA. Clinical spectrum of neuromuscular complications after immune checkpoint inhibition. Neuromuscul Disord. 2019;29(2):127–33. https://doi.org/10.1016/j.nmd.2018.11.012.
• Dubey D, David WS, Amato AA, Reynolds KL, Clement NF, Chute DF, et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology. 2019;93(11):e1093–e103. https://doi.org/10.1212/WNL.0000000000008091. This retrospective case series describes diverse phenotypes of peripheral nervous system pathology derived from two tertiary institutions.
•• Chen X, Haggiagi A, Tzatha E, DeAngelis LM, Santomasso B. Electrophysiological findings in immune checkpoint inhibitor-related peripheral neuropathy. Clin Neurophysiol. 2019;130(8):1440–5. https://doi.org/10.1016/j.clinph.2019.03.035. A retrospective single-institution case series which is the first to describe detailed electrodiagnostic features of ICI-related peripheral neuropathies.
Supakornnumporn S, Katirji B. Guillain-Barre syndrome triggered by immune checkpoint inhibitors: a case report and literature review. J Clin Neuromuscul Dis. 2017;19(2):80–3. https://doi.org/10.1097/CND.0000000000000193.
•• Dubey D, David WS, Reynolds KL, Chute DF, Clement NF, Cohen JV, et al. Severe neurological toxicity of immune checkpoint inhibitors: growing spectrum. Ann Neurol. 2020;87(5):659–69. https://doi.org/10.1002/ana.25708. This study describes all severe neurologic adverse events associated with immune checkpoint inhibitors experienced at a tertiary care institution, including neuromuscular disease as well as overlap syndromes. Moreover, the article includes an in-depth discussion on management.
Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018;37(9):2579–84. https://doi.org/10.1007/s10067-018-4177-0.
Alhammad RM, Dronca RS, Kottschade LA, Turner HJ, Staff NP, Mauermann ML, et al. Brachial plexus neuritis associated with anti-programmed cell death-1 antibodies: report of 2 cases. Mayo Clin Proc Innov Qual Outcomes. 2017;1(2):192–7. https://doi.org/10.1016/j.mayocpiqo.2017.07.004.
Gu Y, Menzies AM, Long GV, Fernando SL, Herkes G. Immune mediated neuropathy following checkpoint immunotherapy. J Clin Neurosci. 2017;45:14–7. https://doi.org/10.1016/j.jocn.2017.07.014.
Hughes RA, Wijdicks EF, Barohn R, Benson E, Cornblath DR, Hahn AF, et al. Practice parameter: immunotherapy for Guillain-Barre syndrome: report of the quality standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61(6):736–40. https://doi.org/10.1212/wnl.61.6.736.
Chen JH, Lee KY, Hu CJ, Chung CC. Coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab in a patient with non-small-cell lung cancer: a case report and literature review. Medicine (Baltimore). 2017;96(50):e9262. https://doi.org/10.1097/MD.0000000000009262.
• Suzuki S, Ishikawa N, Konoeda F, Seki N, Fukushima S, Takahashi K, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89(11):1127–34. https://doi.org/10.1212/WNL.0000000000004359. Describes a series of patients with PD-1 associated myasthenia gravis, detailing the myasthenia – myositis – myocarditis overlap syndrome.
• Liewluck T, Kao JC, Mauermann ML. PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother. 2018;41(4):208–11. https://doi.org/10.1097/CJI.0000000000000196. A case series of PD-1 associated myopathies which describes the unique features of ocular and bulbar involvement as can be seen in ICI-associated myositis.
Kao JC, Brickshawana A, Liewluck T. Neuromuscular complications of programmed cell death-1 (PD-1) inhibitors. Curr Neurol Neurosci Rep. 2018;18(10):63. https://doi.org/10.1007/s11910-018-0878-7.
Kolb NA, Trevino CR, Waheed W, Sobhani F, Landry KK, Thomas AA, et al. Neuromuscular complications of immune checkpoint inhibitor therapy. Muscle Nerve. 2018;58:10–22. https://doi.org/10.1002/mus.26070.
Abdel-Wahab N, Suarez-Almazor ME. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology (Oxford). 2019;58(Suppl 7):vii40–vii8. https://doi.org/10.1093/rheumatology/kez297.
Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheum. 2019;71(3):468–75. https://doi.org/10.1002/art.40745.
• Dalakas MC. Neurological complications of immune checkpoint inhibitors: what happens when you 'take the brakes off' the immune system. Ther Adv Neurol Disord. 2018;11:1756286418799864. https://doi.org/10.1177/1756286418799864. A succinct review article for neurologists describing ICIs, their mechanism of action, proposed reason for autoimmunity, and summarizing all described neurological complications to date of publication.
Uchio NTK, Ikenaga C, Unuma A, Kadoya M, Kubota A, et al. Granulomatous myositis induced by anti-PD-1 monoclonal antibodies. Neurol Neuroimmunol Neuroinflamm. 2018;5(4).
•• Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Aure K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91(10):e985–e94. https://doi.org/10.1212/WNL.0000000000006124. Excellent article that investigates features of patients who develop myositis, including weakness patterns but also histopathology findings.
Anquetil C, Salem JE, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune checkpoint inhibitor-associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation. 2018;138(7):743–5. https://doi.org/10.1161/CIRCULATIONAHA.118.035898.
Diamantopoulos PT, Tsatsou K, Benopoulou O, Anastasopoulou A, Gogas H. Inflammatory myopathy and axonal neuropathy in a patient with melanoma following pembrolizumab treatment. J Immunother. 2017;40(6):221–3. https://doi.org/10.1097/CJI.0000000000000172.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–9. https://doi.org/10.1038/nature22396.
Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol. 2016;29(6):806–12. https://doi.org/10.1097/WCO.0000000000000391.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–68. https://doi.org/10.1200/JCO.2017.77.6385.
Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. https://doi.org/10.1186/s40425-017-0300-z.
Anderson D, Beecher G, Nathoo N, Smylie M, McCombe JA, Walker J, et al. Proposed diagnostic and treatment paradigm for high-grade neurological complications of immune checkpoint inhibitors. Neurooncol Pract. 2019;6(5):340–5. https://doi.org/10.1093/nop/npy039.
Thomson JA SB, Brahmer J, Andrews S, Armand P et al. Managment of immunotherapy-related toxicicites. 2019. https://www.nccn.org/professionals/physician_gls/default.aspx#immunotherapy. Accessed March 6 2020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuromuscular Disorders
Rights and permissions
About this article
Cite this article
Garcia-Santibanez, R., Khoury, M. & Harrison, T.B. Immune-Related Neuromuscular Complications of Checkpoint Inhibitors. Curr Treat Options Neurol 22, 27 (2020). https://doi.org/10.1007/s11940-020-00635-3
Published:
DOI: https://doi.org/10.1007/s11940-020-00635-3