Abstract
Purpose of review
While there are a growing number of therapies targeting relapse prevention in multiple sclerosis (MS), there are no approved therapies promoting remyelination. Understanding endogenous myelin formation, remyelination strategies, pre-clinical models, and clinical outcomes is essential to the interpretation of current and future clinical trials of remyelinating agents.
Recent findings
Several recent clinical trials of remyelination therapies, including opicinumab, clemastine, and GSK239512, showed negative or modest results. These results could highlight challenges translating pre-clinical studies into subjects with MS and current strategies to measure remyelination.
Summary
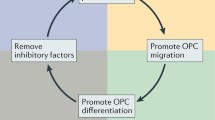
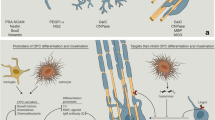
Current approaches to remyelination include (1) blocking inhibitors of remyelination, (2) improving the clearance of myelin debris, (3) increasing the number of oligodendrocyte precursor cells (OPCs), and (4) stimulating OPC differentiation. To date, no therapies have led to robust remyelination. Future efforts to promote remyelination will likely require a combination of these mechanistic strategies.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wang H, et al. miR-219 cooperates with miR-338 in myelination and promotes myelin repair in the CNS. Dev Cell. 2017;40(6):566–582.e5.
Simons M, Nave K-A. Oligodendrocytes: myelination and axonal support. Cold Spring Harbor Perspectives in Biology. 2016;8(1):a020479.
Hughes EG, et al. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neuroscience. 2013;16(6):668–676-676.
• Tsai H-H, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351(6271):379–384-384 This paper shows that oligodendrocyte precursor cells in both mouse brain and human cortex rely upon physical interaction with the vasculature to migrate within the brain. This highlights the complex interactions between cerebral vasculature and oligodendrocytes in creating a permissive environment for myelination.
Lee S, et al. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nature Protocols. 2013;8(4):771.
Rosenberg SS, et al. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proceedings of the National Academy of Sciences. 2008;105(38):14662–14667-14667.
Brinkmann BG, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59(4):581–95.
Schoenemann TP, Sheehan MJ, Glotzer DL. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nature Neuroscience. 2005;8(2):nn1394.
Stassart RM, et al. The axon-myelin unit in development and degenerative disease. Frontiers in Neuroscience. 2018;12:467.
Roth AD, Ivanova A, Colman DR. New observations on the compact myelin proteome. Neuron Glia Biology. 2006;2(1):15–21.
Lee Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443.
Nijland PG, et al. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia. 2014;62(7):1125–1141-1141.
Wu GF, Alvarez E. The immunopathophysiology of multiple sclerosis. Neurologic Clinics. 2011;29(2):257–278-278.
Waxman SG, Craner MJ, Black JA. Na+ channel expression along axons in multiple sclerosis and its models. Trends in Pharmacological Sciences. 2004;25(11):584–591-591.
Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–85.
Kornek B, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. The American Journal of Pathology. 2000;157(1):267–276-276.
Bakshi R, et al. MRI in multiple sclerosis: current status and future prospects. The Lancet Neurology. 2008;7(7):615–625-625.
Fancy S, et al. Myelin regeneration: a recapitulation of development? Annual Review of Neuroscience. 2011;34(1):21 –43-43.
Lucchinetti C, et al. A quantitative analysis of oligodendrocytes in multiple sclerosis lesionsA study of 113 cases. Brain. 1999;122(12):2279–2295-2295.
Popescu BF, et al. Pathology of multiple sclerosis: where do we stand?. Continuum (Minneapolis, Minn.) Lifelong Learning in Neurology. 2013. 19(4, Multiple Sclerosis):901–921. https://doi.org/10.1212/01.CON.0000433291.23091.65
Bramow S, et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133(10):2983–2998-2998.
Stangel M, et al. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nature Reviews Neurology. 2017;13(12):742.
Lundgaard I, et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biology. 2013;11(12):e1001743.
Lee S, et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nature Methods. 2012;9(9):917.
Chong SY, Chan JR. Tapping into the glial reservoir: cells committed to remaining uncommitted. The Journal of cell biology. 2010;188(3):305–12-12.
Gibson EM, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304.
Kotter MR, et al. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. The Journal of Neuroscience. 2006;26(1):328–332-332.
Jensen SK, et al. Multimodal enhancement of remyelination by exercise with a pivotal role for oligodendroglial PGC1alpha. Cell Rep. 2018;24(12):3167–79.
Setzu A, et al. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54(4):297–303-303.
Vallée A, et al. Interactions between the canonical WNT/beta-catenin pathway and PPAR gamma on neuroinflammation, demyelination, and remyelination in multiple sclerosis. Cellular and Molecular Neurobiology. 2018;38(4):783–795-795.
Fancy SP, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23(13):1571–85.
Mi S, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nature Neuroscience. 2005;8(6):745–751-751.
Kuhlmann T, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(7):1749–1758-1758.
Chang A, et al. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–73.
Ciric B, et al. Human monoclonal IgM antibody promotes CNS myelin repair independent of Fc function. Brain Pathology. 2003;13(4):608–616-616.
Huang JK, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nature Neuroscience. 2011;14(1):45.
Buckley CE, et al. Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects. Neuropharmacology. 2010;59(3):149–59.
Mei F, et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med. 2014;20(8):954–60.
•• Green AJ, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial, The Lancet 390.10111. 2017; 2481–2489. This randomized clinical trial of clemastine demonstrated an improvement in VEP after 3–5 months of therapy. This trial demonstrated the potential for remyelination in chronically demyelinated optic nerves.
•• Schwartzbach CJ, et al. Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing-remitting multiple sclerosis: a randomised, single-blind, phase II study. J Neurol. 2017;264(2):304–15 This randomized clinical trial of GSK239512 did not obtain positive results, but did show feasibility for a multicenter trial using MTR as a primary endpoint.
Hall SM. The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J Cell Sci. 1972;10(2):535–46.
Blakemore WF, Franklin RJ. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212.
Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25(3):216–28.
Plemel JR, Liu WQ, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov. 2017;16(9):617–34.
Carlton WW. Studies on the induction of hydrocephalus and spongy degeneration by cuprizone feeding and attempts to antidote the toxicity. Life Sci. 1967;6(1):11–9.
Mason JL, et al. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am J Pathol. 2004;164(5):1673–82.
Doan V, et al. Abbreviated exposure to cuprizone is sufficient to induce demyelination and oligodendrocyte loss. J Neurosci Res. 2013;91(3):363–73.
Miljkovic D, Spasojevic I. Multiple sclerosis: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2013;19(18):2286–334.
Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11(1):107–16.
Baxi EG, et al. Lineage tracing reveals dynamic changes in oligodendrocyte precursor cells following cuprizone-induced demyelination. Glia. 2017;65(12):2087–98.
Kipp M, et al. Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathol. 2017;27(2):123–37.
Gudi V, et al. Glial response during cuprizone-induced de- and remyelination in the CNS: lessons learned. Front Cell Neurosci. 2014;8:73.
Baker D, Amor S. Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult Scler Relat Disord. 2014;3(5):555–64.
Munoz JJ, Bernard CC, Mackay IR. Elicitation of experimental allergic encephalomyelitis (EAE) in mice with the aid of pertussigen. Cell Immunol. 1984;83(1):92–100.
Bjelobaba I, et al. Animal models of multiple sclerosis: focus on experimental autoimmune encephalomyelitis. J Neurosci Res. 2018;96(6):1021–42.
van der Star BJ, et al. In vitro and in vivo models of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11(5):570–88.
Gumpel M, et al. Myelination and remyelination in the central nervous system by transplanted oligodendrocytes using the shiverer model. Discussion on the remyelinating cell population in adult mammals. Dev Neurosci. 1989;11(2):132–9.
Frischer JM, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78(5):710–21.
Shen S, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11(9):1024–34.
Zhao C, Li WW, Franklin RJ. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol Aging. 2006;27(9):1298–307.
Patrikios P, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt 12):3165–72.
Patani R, et al. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33(3):277–87.
Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125(Pt 2):338–49.
Fern R, Moller T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci. 2000;20(1):34–42.
Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol. 1993;111(6):773–5.
Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13(1):83–99.
Matsunaga Y, et al. Visual functional and histopathological correlation in experimental autoimmune optic neuritis. Invest Ophthalmol Vis Sci. 2012;53(11):6964–71.
Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354(9):942–55.
Silbermann E, Wooliscroft L, Bourdette D. Using the anterior visual system to assess neuroprotection and remyelination in multiple sclerosis trials. Curr Neurol Neurosci Rep. 2018;18(8):49.
Klistorner A, Graham SL. Objective perimetry in glaucoma. Ophthalmology. 2000;107(12):2283–99.
Klistorner A, et al. Correlation between full-field and multifocal VEPs in optic neuritis. Doc Ophthalmol. 2008;116(1):19–27.
Brusa A, Jones SJ, Plant GT. Long-term remyelination after optic neuritis: A 2-year visual evoked potential and psychophysical serial study. Brain. 2001;124(Pt 3):468–79.
Weinstock-Guttman B, et al. Pattern reversal visual evoked potentials as a measure of visual pathway pathology in multiple sclerosis. Mult Scler. 2003;9(5):529–34.
Yeung HN, Aisen AM. Magnetization transfer contrast with periodic pulsed saturation. Radiology. 1992;183(1):209–14.
Dousset V, et al. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology. 1992;182(2):483–91.
Schmierer K, et al. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56(3):407–15.
Vavasour IM, et al. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J Magn Reson Imaging. 2011;33(3):713–8.
MacKay A, et al. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med. 1994;31(6):673–7.
Laule C, et al. Myelin water imaging of multiple sclerosis at 7 T: correlations with histopathology. Neuroimage. 2008;40(4):1575–80.
Gareau PJ, et al. Magnetization transfer and multicomponent T2 relaxation measurements with histopathologic correlation in an experimental model of MS. J Magn Reson Imaging. 2000;11(6):586–95.
Le Bihan D, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534–46.
Song SK, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–40.
Cross AH, Song SK. A new imaging modality to non-invasively assess multiple sclerosis pathology. J Neuroimmunol. 2017;304:81–5.
Tran JQ, et al. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurol Neuroimmunol Neuroinflamm. 2014;1(2):e18.
Mi S, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7(3):221–8.
Mi S, Pepinsky RB, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs. 2013;27(7):493–503.
Ranger A, et al. Anti-LINGO-1 has no detectable immunomodulatory effects in preclinical and phase 1 studies. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e417.
Cadavid D, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(3):189–99.
•• Klistorner A, et al. Assessment of opicinumab in acute optic neuritis using multifocal visual evoked potential. CNS Drugs. 2018;32(12):1159–71 This randomized clinical trial of opicinumab (an anti-LINGO antibody) had negative results but demonstrated feasibility of a multisite clinical trial using VEP as a primary outcome.
Bove RM, Green AJ. Remyelinating pharmacotherapies in multiple sclerosis. Neurotherapeutics. 2017;14(4):894–904.
Deshmukh VA, et al. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502(7471):327–32.
Li Z, et al. Clemastine rescues behavioral changes and enhances remyelination in the cuprizone mouse model of demyelination. Neurosci Bull. 2015;31(5):617–25.
Liu J, et al. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci. 2016;36(3):957–62.
Green AJ, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481–9.
Ashworth S, et al. Unexpectedly high affinity of a novel histamine H(3) receptor antagonist, GSK239512, in vivo in human brain, determined using PET. Br J Pharmacol. 2014;171(5):1241–9.
Wilson DM, et al. Identification of clinical candidates from the benzazepine class of histamine H3 receptor antagonists. Bioorg Med Chem Lett. 2013;23(24):6890–6.
Grove RA, et al. A randomized, double-blind, placebo-controlled, 16-week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild-to-moderate Alzheimer’s disease. Curr Alzheimer Res. 2014;11(1):47–58.
Jarskog LF, et al. A phase II study of a histamine H(3) receptor antagonist GSK239512 for cognitive impairment in stable schizophrenia subjects on antipsychotic therapy. Schizophr Res. 2015;164(1–3):136–42.
Chen Y, et al. Histamine receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS One. 2017;12(12):e0189380.
Acknowledgments
Dr. Wooliscroft would like to thank the Veterans Administration MS Center of Excellence-West for their support in her fellowship. Dr. Silbermann would like to thank the National MS Society for their support of her fellowship through a Sylvia Lawry Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lindsey Wooliscroft and Elizabeth Silbermann each declare no potential conflicts of interest. Michelle Cameron reports consulting fees from Adamas Pharmaceuticals and Greenwich Biosciences outside the submitted work. Dennis Bourdette reports consultancy for reviewing patient medical records and providing opinion on treatments for Magellan Health Care and Best Doctors Inc. He also served as an expert witness on MS for the US Department of Justice, reports a bench research grant and collaborative center award from the National MS Society, and reports a founder’s stock valued at $1000 from Llama Therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Multiple Sclerosis and Related Disorders
Rights and permissions
About this article
Cite this article
Wooliscroft, L., Silbermann, E., Cameron, M. et al. Approaches to Remyelination Therapies in Multiple Sclerosis. Curr Treat Options Neurol 21, 34 (2019). https://doi.org/10.1007/s11940-019-0574-1
Published:
DOI: https://doi.org/10.1007/s11940-019-0574-1