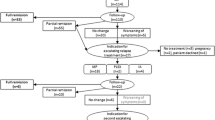
Opinion statement
While not all multiple sclerosis (MS) relapses require treatment, relapses that are bothersome or that impair function should prompt consideration of timely treatment to restore function and minimize disability. Patients with suspected MS relapses should be evaluated to confirm the diagnosis, exclude other causes of neurological dysfunction, and identify potential triggers for relapse or pseudo-relapse, such as urinary tract infections, fever, or metabolic derangements. The diagnosis of an MS relapse is clinical, but MRI may be useful for confirmation and to evaluate for multifocal disease activity. High-dose oral or intravenous glucocorticoids, with or without an oral taper, are first-line therapy for MS relapses. Adrenocorticotropic hormone (ACTH) provides an alternative to glucocorticoid treatment but is currently much more expensive and does not have proven superiority. If the acute neurological deficits remain severe after steroid treatment, and particularly if there is persistent abnormal contrast-enhancement of the symptomatic lesion on repeat MRI, plasma exchange (PLEX) should be considered as an acute rescue therapy for relapse. In exceptional cases, particularly fulminant or tumefactive disease that fails to improve following treatment with steroids and PLEX, cytoxic agents such as cyclophosphamide or B cell-depleting regimens such as rituximab may be considered, although risk must be carefully weighed and the kinetics of such regimens indicate that they probably serve more to accelerate remission of disease activity than as an immediate relapse remedy. A single dose of natalizumab given as acute therapy for MS relapse was shown not to improve clinical outcomes in a randomized controlled trial. Attention to symptom management and promotion of neurorehabilitation are important aspects of MS relapse care. Neuroprotective and neuroreparative therapies remain under investigation, but are likely to become important complementary elements of relapse therapy in the future. Relapses serve as important indicators of MS disease activity. In the context of the emerging treatment paradigm of targeting freedom from evidence of MS disease activity, relapses should prompt consideration of transitioning to a disease-modifying treatment that may offer better efficacy.

Similar content being viewed by others
References and Recommended Reading
Gelfand JM. Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. Handb Clin Neurol. 2014;122:269–90.
Weinshenker BG et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain. 1989;112(Pt 6):1419–28.
Polman CH et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.
Scalfari A et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914–29.
Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(Pt 4):770–82.
Balashov KE, Lindzen E. Acute demyelinating lesions with restricted diffusion in multiple sclerosis. Mult Scler. 2012;18(12):1745–53.
Gaitan MI et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70(1):22–9.
Horsfield MA et al. Diffusion magnetic resonance imaging in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64 Suppl 1:S80–4.
Simon JH. MRI outcomes in the diagnosis and disease course of multiple sclerosis. Handb Clin Neurol. 2014;122:405–25.
Isaac C et al. Multiple sclerosis: a serial study using MRI in relapsing patients. Neurology. 1988;38(10):1511–5.
Green AJ. Understanding pseudo: the symptoms are real, the cause is unclear. Neurology. 2009;72(19):1626–7.
Thrower BW. Relapse management in multiple sclerosis. Neurologist. 2009;15(1):1–5.
Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann Neurol. 1994;36(Suppl):S25–8.
Trapp BD et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–85.
Ramo-Tello C et al. A randomized clinical trial of oral versus intravenous methylprednisolone for relapse of MS. Mult Scler. 2014;20(6):717–25.
Filippini G et al. Corticosteroids or ACTH for acute exacerbations in multiple sclerosis. Cochrane Database Syst Rev. 2000;4, CD001331.
Berkovich R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics. 2013;10(1):97–105.
Ross AP, Halper J, Harris CJ. Assessing relapses and response to relapse treatment in patients with multiple sclerosis: a nursing perspective. Int J MS Care. 2012;14(3):148–59.
Thompson AJ et al. Relative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MS. Neurology. 1989;39(7):969–71.
Glaser GH, Merritt HH. Effects of ACTH and cortisone in multiple sclerosis. Trans Am Neurol Assoc. 1951;56:130–3.
Magana SM et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011;68(7):870–8.
Weinshenker BG et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878–86.
Weiner HL et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology. 1989;39(9):1143–9.
O’Connor PW et al. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology. 2004;62(11):2038–43.
Weiner HL. Immunosuppressive treatment in multiple sclerosis. J Neurol Sci. 2004;223(1):1–11.
Edan G et al. Mitoxantrone prior to interferon beta-1b in aggressive relapsing multiple sclerosis: a 3-year randomised trial. J Neurol Neurosurg Psychiatry. 2011;82(12):1344–50.
Coles AJ. Alemtuzumab treatment of multiple sclerosis. Semin Neurol. 2013;33(1):66–73.
Pfender N, Saccardi R, Martin R. Autologous hematopoietic stem cell transplantation as a treatment option for aggressive multiple sclerosis. Curr Treat Options Neurol. 2013;15(3):270–80.
Burman J et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014;85(10):1116–21.
Freedman MS. ‘Time is brain’ also in multiple sclerosis. Mult Scler. 2009;15(10):1133–4.
Sorensen PS. New management algorithms in multiple sclerosis. Curr Opin Neurol. 2014;27(3):246–59.
Okuda DT. Immunosuppressive treatments in multiple sclerosis. Handb Clin Neurol. 2014;122:503–11.
O’Connor PW, Oh J. Disease-modifying agents in multiple sclerosis. Handb Clin Neurol. 2014;122:465–501.
Beck RW et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326(9):581–8.
Goodin DS. Perils and pitfalls in the interpretation of clinical trials: a reflection on the recent experience in multiple sclerosis. Neuroepidemiology. 1999;18(2):53–63.
Miller H, Newell DJ, Ridley A. Multiple sclerosis. Treatment of acute exacerbations with corticotrophin (A.C.T.H.). Lancet. 1961;2(7212):1120–2.
Rose AS et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo—final report. Neurology. 1970;20(5):1–59.
Sellebjerg F et al. Randomized controlled trial of high-dose peroral methylprednisolone in attacks of multiple sclerosis. Ugeskr Laeger. 1999;161(48):6625–9.
Sellebjerg F et al. Double-blind, randomized, placebo-controlled study of oral, high-dose methylprednisolone in attacks of MS. Neurology. 1998;51(2):529–34.
Filipovic SR et al. The effects of high-dose intravenous methylprednisolone on event-related potentials in patients with multiple sclerosis. J Neurol Sci. 1997;152(2):147–53.
Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. Clinical effects. J Neurol Neurosurg Psychiatry. 1987;50(5):511–6.
Durelli L et al. High-dose intravenous methylprednisolone in the treatment of multiple sclerosis: clinical-immunologic correlations. Neurology. 1986;36(2):238–43.
Morrow SA et al. The bioavailability of IV methylprednisolone and oral prednisone in multiple sclerosis. Neurology. 2004;63(6):1079–80.
Martinelli V et al. A short-term randomized MRI study of high-dose oral vs intravenous methylprednisolone in MS. Neurology. 2009;73(22):1842–8.
Barnes D et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet. 1997;349(9056):902–6.
Piper JM et al. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;114(9):735–40.
Arvin AM et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 2015;72(1):31–9.
Arnason BG et al. Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler. 2013;19(2):130–6.
U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Application number 022432Orig1s000 Risk Assessment and Risk Mitigation Review(s). 2010. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022432Orig1s000RiskR.pdf Accessed 3 March 2015.
Pollack A. Questcor finds profits at $28,000 a vial. The New York Times. Published December 29, 2012. http://www.nytimes.com/2012/12/30/business/questcor-finds-profit-for-acthar-drug-at-28000-a-vial.html Accessed 3 March 2015.
Keegan M et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58(1):143–6.
Llufriu S et al. Plasma exchange for acute attacks of CNS demyelination: predictors of improvement at 6 months. Neurology. 2009;73(12):949–53.
Sorensen PS et al. IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS. Neurology. 2004;63(11):2028–33.
Visser LH et al. A randomized, double-blind, placebo-controlled pilot study of i.v. immune globulins in combination with i.v. methylprednisolone in the treatment of relapses in patients with MS. Mult Scler. 2004;10(1):89–91.
Roed HG et al. A double-blind, randomized trial of IV immunoglobulin treatment in acute optic neuritis. Neurology. 2005;64(5):804–10.
Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007;13(7):900–8.
Achiron A et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004;251(9):1133–7.
Achiron A et al. Intravenous immunoglobulin treatment in multiple sclerosis and experimental autoimmune encephalomyelitis—the Israeli experience. MS Study Group. Mult Scler. 1997;3(2):142–4.
Fazekas F et al. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis. Austrian Immunoglobulin in Multiple Sclerosis Study Group. Lancet. 1997;349(9052):589–93.
Elovaara I et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol. 2008;15(9):893–908.
Goodin DS et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58(2):169–78.
Fazekas F et al. Intravenous immunoglobulin in relapsing-remitting multiple sclerosis: a dose-finding trial. Neurology. 2008;71(4):265–71.
Siffrin V et al. How to treat tumefactive demyelinating disease? Mult Scler. 2014;20(5):631–3.
Sempere AP et al. Neurological picture. Rituximab for tumefactive demyelination refractory to corticosteroids and plasma exchange. J Neurol Neurosurg Psychiatry. 2013;84(12):1338–9.
Fan X et al. Rituximab for tumefactive inflammatory demyelination: a case report. Clin Neurol Neurosurg. 2012;114(10):1326–8.
Hardy TA, Chataway J. Tumefactive demyelination: an approach to diagnosis and management. J Neurol Neurosurg Psychiatry. 2013;84(9):1047–53.
Harrison DM et al. Treatment of relapsing-remitting multiple sclerosis with high-dose cyclophosphamide induction followed by glatiramer acetate maintenance. Mult Scler. 2012;18(2):202–9.
Krishnan C et al. Reduction of disease activity and disability with high-dose cyclophosphamide in patients with aggressive multiple sclerosis. Arch Neurol. 2008;65(8):1044–51.
Makhani N et al. Cyclophosphamide therapy in pediatric multiple sclerosis. Neurology. 2009;72(24):2076–82.
Smith DR et al. A randomized blinded trial of combination therapy with cyclophosphamide in patients-with active multiple sclerosis on interferon beta. Mult Scler. 2005;11(5):573–82.
Le Page E et al. Long-term safety profile of mitoxantrone in a French cohort of 802 multiple sclerosis patients: a 5-year prospective study. Mult Scler. 2011;17(7):867–75.
Azzopardi L et al. Predicting autoimmunity after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(7):795–8.
Bevan CJ, Cree BA. Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA Neurol. 2014;71(3):269–70.
Havrdova E et al. Disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with daclizumab high-yield process in the SELECT study. Mult Scler. 2014;20(4):464–70.
Giovannoni G et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10(4):329–37.
Vercellino M et al. Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand. 2009;119(2):126–30.
Noseworthy JH et al. Multiple sclerosis. N Engl J Med. 2000;343(13):938–52.
van Winsen LM et al. Outcome measurement in multiple sclerosis: detection of clinically relevant improvement. Mult Scler. 2010;16(5):604–10.
Solaro C, Uccelli MM. Management of pain in multiple sclerosis: a pharmacological approach. Nat Rev Neurol. 2011;7(9):519–27.
Hadjimichael O et al. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007;127(1–2):35–41.
Tang DH et al. Impact of urinary incontinence on health-related quality of life, daily activities, and healthcare resource utilization in patients with neurogenic detrusor overactivity. BMC Neurol. 2014;14:74.
Rizzo MA et al. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004;10(5):589–95.
Zwibel HL, Smrtka J. Improving quality of life in multiple sclerosis: an unmet need. Am J Manag Care. 2011;17(Suppl 5 Improving):S139–45.
Frohman TC et al. Symptomatic therapy in multiple sclerosis. Ther Adv Neurol Disord. 2011;4(2):83–98.
Courtney AM et al. Functional treatments in multiple sclerosis. Curr Opin Neurol. 2011;24(3):250–4.
Craig J et al. A randomised controlled trial comparing rehabilitation against standard therapy in multiple sclerosis patients receiving intravenous steroid treatment. J Neurol Neurosurg Psychiatry. 2003;74(9):1225–30.
Hauser SL, Chan JR, Oksenberg JR. Multiple sclerosis: prospects and promise. Ann Neurol. 2013;74(3):317–27.
Suhs KW et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72(2):199–210.
Acknowledgments
Funding was provided through the National MS Society Institutional Clinician Training Award and NIH KL2TR000143.
Compliance with Ethics Guidelines
Conflict of Interest
Carolyn Bevan declares no conflict of interest.
Jeffrey M. Gelfand declares that he has received compensation for medical legal consulting relating to CNS inflammatory disease. Dr. Gelfand has also received compensation for consulting for MedImmune and Quest Diagnostics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Multiple Sclerosis and Related Disorders
Rights and permissions
About this article
Cite this article
Bevan, C., Gelfand, J.M. Therapeutic Management of Severe Relapses in Multiple Sclerosis. Curr Treat Options Neurol 17, 17 (2015). https://doi.org/10.1007/s11940-015-0345-6
Published:
DOI: https://doi.org/10.1007/s11940-015-0345-6