Abstract
Purpose of review
To critically examine recently published research in the area of chemoprevention in hereditary polyposis and gastrointestinal cancers, and to briefly review several ongoing chemoprevention trials testing novel agents in this population.
Recent findings
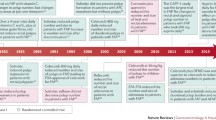
Four recent chemoprevention trials in patients with familial adenomatous polyposis (FAP) were identified and reviewed. In the FAPEST trial, the combination of erlotinib + sulindac (compared to placebo) met its primary outcome of decreased duodenal polyp burden. A secondary analysis of lower gastrointestinal tract outcomes also demonstrated significant benefits. Two randomized trials in FAP patients examining combination regimens (celecoxib + DFMO and sulindac + DFMO) failed to meet their primary endpoints. Benefits of further research into these combinations were suggested by efficacy signals seen in secondary and post hoc analyses. Finally, a randomized trial found curcumin (versus placebo) to have no benefit in reducing colorectal polyp count or size in patients with FAP.
Summary
Progress in developing new and more effective preventive options for patients with hereditary gastrointestinal syndromes continues to be made through the efforts of investigators conducting chemoprevention research.
Trial registration
NCT02961374, NCT03333265, NCT03649971, NCT04296851, NCT03806426, NCT04230499, NCT01725490, CaPP3 trial, NCT02813824, NCT03831698, NCT04379999, NCT02052908

Similar content being viewed by others
Change history
08 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11938-021-00332-3
References and Recommended Reading
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin.
Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, et al. (eds). SEER cancer statistics review https://seer.cancer.gov/csr/1975_2017/. 1975–2017 [updated April 2020. Available from: https://seer.cancer.gov/csr/1975_2017/.
Joseph DA, King JB, Richards TB, Thomas CC, Richardson LC. Use of colorectal cancer screening tests by state. Prev Chronic Dis. 2018;15:E80.
Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–27.
Chubak J, Whitlock EP, Williams SB, Kamineni A, Burda BU, Buist DS, et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. preventive services task force. Ann Intern Med. 2016;164(12):814–25.
Rothwell PM, Wilson M, Elwin C-E, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–50.
Ajani UA, Ford ES, Greenland KJ, Giles WH, Mokdad AH. Aspirin use among U.S. adults: behavioral risk factor surveillance system. Am J Prev Med. 2006;30(1):74–7.
Fiedler B, Fiedler L, DeDonno M, Anago K, de la Cruz L, Luck GR, et al. Underutilization of aspirin in patients with advanced colorectal polyps. Am J Med. 2019;132(7):884–5.
Kidambi TD, Kohli DR, Samadder NJ, Singh A. Hereditary polyposis syndromes. Curr Treat Options Gastroenterol. 2019;17(4):650–65.
Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am. 2009;18(4):585–98.
Sinicrope FA. Lynch syndrome-associated colorectal cancer. N Engl J Med. 2018;379(8):764–73.
Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomark Prev. 2017;26(3):404–12.
Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila). 2011;4(5):655–65.
Burn J, Bishop DT, Mecklin J-P, Macrae F, Möslein G, Olschwang S, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359(24):2567–78.
www.capp3.org. CANCER PREVENTION WITH ASPIRIN: FACT SHEET.
Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–6.
Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346(14):1054–9.
Nugent KP, Farmer KCR, Spigelman AD, Williams CB, Phillips RKS. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80(12):1618–9.
Higuchi T, Iwama T, Yoshinaga K, Toyooka M, Taketo MM, Sugihara K. A randomized, double-blind, placebo-controlled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin Cancer Res. 2003;9(13):4756–60.
Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, et al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50(6):857–60.
Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52.
van Heumen BWH, Roelofs HMJ, Vink-Börger ME, Dekker E, Mathus-Vliegen EMH, Dees J, et al. Ursodeoxycholic acid counteracts celecoxib in reduction of duodenal polyps in patients with familial adenomatous polyposis: a multicentre, randomized controlled trial. Orphanet J Rare Dis. 2013;8(1):118.
Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–7.
West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, Belluzzi A, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59(7):918–25.
Parc Y, Desaint B, Fléjou JF, Lefèvre JH, Serfaty L, Vienne A, et al. The effect of ursodesoxycholic acid on duodenal adenomas in familial adenomatous polyposis: a prospective randomized placebo-control trial. Color Dis. 2012;14(7):854–60.
Ramamurthy C, Chertock Y, Hall MJ. Randomized controlled trials in hereditary Cancer syndromes. Surg Oncol Clin N Am. 2017;26(4):729–50.
Backlund MG, Mann JR, DuBois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69(suppl 1):28–32.
Tomić T, Domínguez-López S, Barrios-Rodríguez R. Non-aspirin non-steroidal anti-inflammatory drugs in prevention of colorectal cancer in people aged 40 or older: a systematic review and meta-analysis. Cancer Epidemiol. 2019;58:52–62.
Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101(3):635–9.
Baron JA, Greenberg ER. Could aspirin really prevent colon cancer? N Engl J Med. 1991;325(23):1644–6.
Paganini-Hill A, Hsu G, Ross RK, Henderson BE. Aspirin use and incidence of large-bowel cancer in a California retirement community. J Natl Cancer Inst. 1991;83(16):1182–3.
Moran AE, Hunt DH, Javid SH, Redston M, Carothers AM, Bertagnolli MM. Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6J-Min/+ mice. J Biol Chem. 2004;279(41):43261–72.
Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci. 2002;99(3):1521–6.
Samadder NJ, Neklason DW, Boucher KM, Byrne KR, Kanth P, Samowitz W, et al. Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: a randomized clinical trial. JAMA. 2016;315(12):1266–75.
Samadder NJ, Kuwada SK, Boucher KM, Byrne K, Kanth P, Samowitz W, et al. Association of sulindac and erlotinib vs placebo with colorectal neoplasia in familial adenomatous polyposis: secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(5):671–7.
Lynch PM, Burke CA, Phillips R, Morris JS, Slack R, Wang X, et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut. 2016;65(2):286–95.
Cruz-Correa M, Hylind LM, Marrero JH, Zahurak ML, Murray-Stewart T, Casero RA Jr, et al. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology. 2018;155(3):668–73.
Burke CA, Dekker E, Samadder NJ, Stoffel E, Cohen A. Efficacy and safety of eflornithine (CPP-1X)/sulindac combination therapy versus each as monotherapy in patients with familial adenomatous polyposis (FAP): design and rationale of a randomized, double-blind, Phase III trial. BMC Gastroenterol. 2016;16(1):87.
Burke CA, Dekker E, Lynch P, Samadder NJ, Balaguer F, Hüneburg R et al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. N Engl J Med. 2020;10;383(11):1028–1039.
Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–95.
Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–82.
Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–84.
Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–102.
Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–80.
Jacoby RF, Cole CE, Tutsch K, Newton MA, Kelloff G, Hawk ET, et al. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000;60(7):1864–70.
Li H, Schut HAJ, Conran P, Kramer PM, Lubet RA, Steele VE, et al. Prevention by aspirin and its combination with α-difluoromethylornithine of azoxymethane-induced tumors, aberrant crypt foci and prostaglandin E2 levels in rat colon. Carcinogenesis. 1999;20(3):425–30.
Tempero MA, Nishioka K, Knott K, Zetterman RK. Chemoprevention of mouse colon tumors with difluoromethylornithine during and after carcinogen treatment. Cancer Res. 1989;49(21):5793–7.
Meyskens FL, Gerner EW, Emerson S, Pelot D, Durbin T, Doyle K, et al. Effect of α-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst. 1998;90(16):1212–8.
Meyskens FL Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila). 2008;1(1):32–8.
Willenbacher E, Khan SZ, Mujica SCA, Trapani D, Hussain S, Wolf D, et al. Curcumin: new insights into an ancient ingredient against cancer. Int J Mol Sci. 2019;20(8).
Murray-Stewart T, Dunworth M, Lui Y, Giardiello FM, Woster PM, Casero RA Jr. Curcumin mediates polyamine metabolism and sensitizes gastrointestinal cancer cells to antitumor polyamine-targeted therapies. PLoS One. 2018;13(8):e0202677.
Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4(8):1035–8.
clinicaltrials.gov. 2020 [Available from: https://clinicaltrials.gov/ct2/results?cond=Colorectal+Cancer&term=&cntry=&state=&city=&dist=.
Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019;45:1–8.
Ahn SY, Kim NH, Lee K, Cha YH, Yang JH, Cha SY, et al. Niclosamide is a potential therapeutic for familial adenomatosis polyposis by disrupting Axin-GSK3 interaction. Oncotarget. 2017;8(19):31842–55.
Hull MA, Sprange K, Hepburn T, Tan W, Shafayat A, Rees CJ, et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod polyp prevention trial): a multicentre, randomised, double-blind, placebo-controlled, 2 × 2 factorial trial. Lancet. 2018;392(10164):2583–94.
Hardiman KM, Liu J, Feng Y, Greenson JK, Fearon ER. Rapamycin inhibition of polyposis and progression to dysplasia in a mouse model. PLoS One. 2014;9(4):e96023.
Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–5.
Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475–83.
Yates CM, Calder PC, Ed RG. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141(3):272–82.
Liu Y, Tang W, Wang J, Xie L, Li T, He Y, et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control. 2014;25(2):237–49.
Lochhead P, Chan AT. Statins and colorectal cancer. Clin Gastroenterol Hepatol. 2013;11(2):109–18.
Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J Gastroenterol. 2014;20(7):1858–70.
Ait Ouakrim D, Dashti SG, Chau R, Buchanan DD, Clendenning M, Rosty C, et al. Aspirin, ibuprofen, and the risk of colorectal cancer in Lynch syndrome. J Natl Cancer Inst. 2015;107(9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genetics in Gastroenterology Practice
Rights and permissions
About this article
Cite this article
Hall, M.J. Updates in Chemoprevention Research for Hereditary Gastrointestinal and Polyposis Syndromes. Curr Treat Options Gastro 19, 30–46 (2021). https://doi.org/10.1007/s11938-020-00306-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-020-00306-x