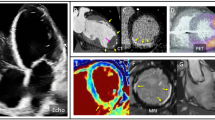
Abstract
Purpose of review
Ventricular arrhythmias, including ventricular tachycardia (VT), ventricular fibrillation (VF), and premature ventricular complexes (PVCs), may occur in structurally normal hearts and in the context of structural heart disease. In those patients with recurrent arrhythmias despite medical therapy, catheter ablation may be considered. To successfully suppress ventricular arrhythmias, an understanding of the substrate for the arrhythmias is crucial.
Recent findings
Advances in cross-sectional imaging used prior to VT ablation permit accurate localisation of fibrosis that represents the substrate for VT, allowing an operator to focus the electrophysiologic assessment during a procedure and effectively target all relevant parts of the substrate. In addition, the use of imaging during a procedure allows registration of pre-procedural cross-sectional imaging as well as real-time substrate assessment and allows the operator to visualise tissue-catheter contact for the most effective lesion delivery.
Summary
In this review, the role of pre-procedural cardiac computed tomographic (CCT) imaging and cardiovascular magnetic resonance (CMR) imaging and the peri-procedural use of intra-cardiac echocardiography (ICE) are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Opinion statement
Ventricular arrhythmias may occur in structurally normal hearts or those with structural abnormalities which may range from minor abnormalities to advanced cardiomyopathies. Fibrotic change within the heart is the most common structural abnormality that is associated with ventricular arrhythmias. Where fibrosis is identified, it may be distributed in any pattern. Ventricular structures also demonstrate significant variability from cavity shape and size to the location and distribution of intra-cavity structures. These considerations represent challenges when attempting to localise the source of VT, navigate to that source, and effectively deliver treatment. Contemporary cross-sectional imaging permits the detailed pre-procedural characterisation of both the structure of the ventricles and the distribution of fibrosis, allowing electrophysiologists to approach ablation procedures with a greater than ever understanding of the anatomic substrate for the arrhythmia. Pre-procedural understanding of the substrate should permit the operator to carefully plan chamber access, likely targets, and ablation energies effectively. The effective combination of pre-procedural and peri-procedural information allows the operator to leverage detailed substrate information from scar imaging in the same image space as the electrophysiologic information. This provides the operator with real-time insight into the structure of the tissue being assessed and treated. In addition, it develops the operator’s understanding of the relationship between the structural and electrophysiologic data, important for both treating individual patients and also advancing our understanding of this relationship in a broader sense. With these considerations in mind, the use of high-fidelity pre- and peri-procedural cardiac imaging is an invaluable tool in the advancement of treatment of ventricular arrhythmias, and the expanded use of these imaging tools will contribute to the development of understanding the complex interaction between substrate and arrhythmia.
Introduction
Sudden cardiac death (SCD) accounts for approximately half of all cardiovascular deaths worldwide, of which a significant proportion is secondary to malignant ventricular arrhythmias (VA) [1]. VA including premature ventricular complexes (PVC), ventricular tachycardia (VT), and ventricular fibrillation (VF) most often occur in the setting of structural heart disease (SHD) but can also occur in structurally normal hearts. Patients resuscitated from SCD and those considered at high risk of it may be offered implantable cardioverter-defibrillators (ICDs) [2], and in those who experience recurrent ICD therapies despite the use of anti-arrhythmic drugs, catheter ablation (CA) may be considered. Prior to undertaking interventional procedures to target VA, it is valuable to gather as much diagnostic information about the substrate as possible, as this may inform the ablation strategy, access considerations, and pre-procedural counselling. A wide variety of imaging techniques are available pre-procedurally to identify the presence of SHD and, in the case of structurally abnormal hearts, to identify the location of myocardial fibrosis, which represents the tissue substrate for the majority of VA. Making full use of the value of pre-procedural imaging allows an operator to plan a procedural approach best suited to the location and extent of any substrate identified. With the emergence of non-invasive ablation modalities, including cardiac stereotactic body radiation (cSBRT) therapy, where it may be necessary to plan therapy without the benefit of invasive electrophysiologic information, substrate delineation is of critical importance. In this review, the indications, benefits, and limitations of cardiovascular magnetic resonance (CMR), cardiac computed tomographic (CCT), and intra-cardiac echocardiography (ICE) will be discussed with specific reference to their application as pre- and peri-procedural imaging modalities.
Cardiovascular magnetic resonance
Ventricular CMR imaging is the reference standard non-invasive technique for assessment of cardiac chamber volumes, systolic function, and tissue characterisation. Late-gadolinium enhancement (LGE)-CMR is the most widely used tissue characterisation application for ventricular myocardium and has a central role in the identification of myocardial scar [3]. CMR does not expose the patient to ionising radiation or iodine-based contrast agents. In a canine model of myocardial infarction, when compared against a nuclear viability tracer, a gadolinium-based contrast agent (GBCA) accumulated in non-viable areas of infarcted myocardium when assessed using ex vivo CMR [4]. In vivo, LGE-CMR in rat [5], rabbit [6], and canine [7] models have also validated the use of LGE-CMR to identify regions corresponding to histological scar tissue. LGE-CMR has been assessed using ultra-high resolution ex vivo imaging, which demonstrates that GBCA localise to post-myocardial infarction (MI) scar at a near cellular level [8]. The utility of LGE-CMR in the identification of myocardial scar has also been comprehensively demonstrated in clinical use, where it can satisfactorily distinguish viable and non-viable myocardium [9, 10], and has become widely used to guide revascularization decisions [11].
Pre-procedural imaging prior to VT ablation offers a range of benefits including prognostic information [12], diagnostic refinement in patients with VA [13], localisation of substrate to guide procedural access [14], identification of global markers of susceptibility to arrhythmia [15, 16], and the ability to identify specific regions harbouring arrhythmogenic features that can be targeted for ablation [17].
The presence of ventricular scar identified using LGE-CMR is associated with an increased risk of SCD, recurrent VT, and appropriate ICD discharge in patients with impaired LV systolic function, regardless of aetiology [12, 18]. LGE-CMR accurately identifies fibrosis in both ischaemic and non-ischaemic cardiomyopathies, and the distribution of scar may be diagnostic for disease process [19].
In addition to providing information about the overall susceptibility to arrhythmias, CMR has been applied to compare local tissue characteristics and specific components of electrophysiologically defined re-entrant VT circuits. Critical isthmus sites are typically found close (< 5 mm) to areas of LGE-CMR defined scar of > 75% transmurality and to the transition between heterogeneous tissue (HT) and dense scar (defined according to 35% and 50% of maximum signal intensity, respectively) [20]. Other reports have localised critical sites of the VT circuit (defined as entrance, central, and exit) to areas of > 25% transmurality and central isthmus sites to areas of > 75% transmurality on LGE-CMR as well as having localised isthmus sites to areas of decreased wall thickness [17]. Higher resolution LGE-CMR has been reported to demonstrate the close correspondence between areas of HT on LGE-CMR and conducting channels identified on electro-anatomic mapping (EAM) [21].
The identification of ‘conducting channels’ (CC) from LGE-CMR as an adjunct to VT ablation has been pioneered in Barcelona [22]. The approach involves the classification of myocardial tissue as a scar, heterogeneous tissue (HT), or non-scar by application of a variable signal-intensity (SI) threshold algorithm (60 ± 5/40 ± 5% of total SI window within the image) to identify dense scar and HT, respectively. Regions of preserved viability are then filtered according to their relationship to other regions of preserved viability to identify areas of non-transmural scar, which harbour anatomic connections between healthy tissue. On a segmental basis, such an approach has not only successfully identified up to 80% of intermediate voltage-defined [22] conducting channels [22] and 74% of VT isthmus locations [21], but its implementation into a clinical programme of VT ablation (as an adjunct to electrophysiologically guided ablation rather than as the primary guide to delivery of ablation therapy) facilitated the delivery of less RF while improving procedural and medium term endpoints following VT ablation [23]. By separation of the myocardial wall into discrete layers (10–25%, 25–50%, 50–75%, and 75–90%), the intramural location of the conducting channels has been defined, which has contributed information about the location within the myocardial wall of the structural substrate responsible for clinical post-MI VT [21]. Taking non-transmural scar/HT as the marker for arrhythmogenic tissue, the imaging acquired in this experiment identified that more than 80% of diastolic isthmus locations were situated in regions of non-transmural scar/HT on a point-by-point basis.
While CMR is the reference standard for scar imaging in the ventricle, the diagnostic utility is often limited by the presence of cardiac implantable electrical devices (CIED), which the majority of patients undergoing VT ablation have in situ. While the safety concerns regarding CMR imaging of patients with CIEDs have been largely overcome with the use of particular protocols and empiric evidence of the safety of acquiring such imaging [24, 25], as well as the emergence of CIEDs designed with consideration of the MR environment [26], the artefact caused by the presence of devices continues to represent a barrier to comprehensive diagnostic ventricular imaging in some patients. These considerations motivated the development of dedicated ‘wide-band’ CMR techniques, which are specifically designed to minimise the artefact caused by the presence of CIEDs [27]. These techniques have improved the ability of CMR imaging to provide diagnostic information regarding ventricular fibrosis in patients with CIED, and the utility of such imaging in identifying the electrophysiologically relevant substrate has been demonstrated (Fig. 1) [28]. An example of a 3D model derived from 3D LGE-CMR indicating scar transmurality is shown in Fig. 2.
In vivo late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) imaging. A–C Short (A) and long (B/C) axis views of 3D LGE-CMR showing heterogeneity within scar tissue including areas of sub-endocardial, mid-myocardial, and sub-epicardial preserved viability. There is no ICD present in this patient which facilitated artefact-free imaging. D 2D 4-chamber LGE-CMR in a patient with a subcutaneous ICD present. The mid to apical septum and lateral walls of the left ventricle are not diagnostically imaged due to artefact from the device. E Wide-band LGE-CMR from the same patient as panel demonstrating much-reduced artefact from device and permitting greater extent of diagnostic imaging. F–H 3D wide-band LGE CMR in the same patient as D/F demonstrating ring pattern epicardial to mid-wall fibrosis within minimal residual device-associated artefacts. Yellow, blue, and red lines indicate the multi-planar reconstruction planes in this imaging.
A Short-axis late gadolinium enhancement-cardiovascular magnetic resonance (LGE-CMR) imaging at 4 short-axis levels indicating anteroseptal scar of heterogeneous transmurality. B 3D endocardial shell derived from 3D LGE-CMR imaging colour-coded according to scar transmurality. Planes indicate the level of CMR imaging from A.
LGE-CMR is the reference standard modality for ventricular tissue characterisation and has been shown to be a valuable tool for guiding VT ablation procedures. The image quality is vulnerable to artefact in the near-ubiquitous presence of CIED in the population of patients undergoing VT ablation in the context of SHD (Fig. 1D). To some extent, this limitation may be overcome through the use of wide-band inversion pulses, increased receiver bandwidth, and the use of spoiled gradient echo rather than balanced steady-state precession sequences to reduce this artefact. Further experience with these sequences and increased availability of this technology may allow the benefit of LGE-CMR to be extended further in the future.
Cardiac CT
Cardiac computed tomographic (CCT) imaging is a widely available tool that may provide structural information that is valuable when planning invasive ablation procedures [29]. CCT typically offers a higher spatial resolution in comparison to CMR and isotropic imaging, however, provides a lower contrast-to-noise ratio for soft-tissue differentiation, and exposes the patient to ionising radiation and potentially nephrotoxic iodine-based contrast agents [30]. CCT angiography may accurately delineate the course of the coronary vessels and identify coronary stenoses that may be a relevant trigger for arrhythmia, which when identified may prompt consideration of revascularization as a strategy to control ventricular arrhythmia [31]. Furthermore, an appreciation of the coronary anatomy is crucial prior to epicardial procedures, and an epicardial access strategy may be directly planned following a review of extra-cardiac anatomy derived from CT [32]. CT imaging can be integrated into electro-anatomic mapping (EAM) systems to allow real-time appreciation of the anatomical relationships between critical cardiac and extra-cardiac structures to ablation targets [33, 34].
In addition to the coronary anatomy, advanced CCT techniques may also identify the location and extent of substrate amongst patients undergoing VT ablation in the context of SHD. Prior myocardial infarction is readily identified as regions of wall thinning and regional wall motion abnormalities, which may be appreciated, along with accurate chamber volume and systolic function, with the use of full retrospective, ECG-gated CCT, which further complements the identification of scar (Fig. 3) [35]. In addition, infarcts may be appreciated as regions of hypoperfusion within the myocardium, identified as regions of reduced attenuation (quantified in the Hounsfield Units) compared to surrounding myocardium [36]. Furthermore, regions of fatty metaplasia and calcification within historic infarcts are readily appreciated with CCT, which may differentiate these particular types of ventricular remodelling with greater sensitivity than CMR [37]. Wall thinning (typically assessed at 2 or 5-mm thresholds) has been shown to co-localise with regions of electrophysiologically defined substrate on EAM during invasive procedures (examples Fig. 3A, B) [38]. This may allow operators to focus electrophysiologic assessment on the regions of CCT identified scar and potentially reduce procedural duration. Furthermore, regions of preserved tissue thickness surrounded by thinned tissue, sensitively (although less specifically), identified isthmuses of conduction supporting post-infarction VT [39,40,41]. Wall thinning is a less sensitive parameter for identifying arrhythmogenic channels of conduction in non-ischemic cardiomyopathy (NICM) [42]. In this context, direct tissue characterisation using late-iodine enhancement (LIE) CCT imaging may be more helpful [43]. LIE CCT was developed from the same principle as LGE-CMR. Briefly, in the late phase following injection of iodine-based contrast media, contrast is distributed throughout the extracellular space. Regions of fibrosis, in which there is an increased proportion of extra-cellular space within a given volume of tissue when compared to healthy tissue, will therefore contain more iodine per unit volume of tissue, resulting in differential photon-absorption behaviour, which can therefore be used to differentiate between tissue types. Regions of fibrosis therefore show greater enhancement than neighbouring healthy tissue. This has been shown to be useful in the identification of regions of fibrosis in both the ischemic [44, 45] and non-ischemic [46•] cardiomyopathies and again has the benefit of allowing the operator to carefully plan an ablation strategy to best access the substrate during the procedure (example Fig. 3C). In an attempt to more precisely and objectively characterise regions of fibrosis, techniques to quantitatively assess density of fibrosis have been developed, which estimate the extra-cellular tissue volume [47, 48]. This has the value of identifying the myocardial level (subendocardial, mid-myocardial, or sub-epicardial) of the substrate as well as the detection of diffuse fibrosis suggestive of generalised cardiomyopathic states, which will provide important prognostic information relevant to accurate pre-procedural counselling [49]. This strategy represents an important development, as a binary classification strategy labelling tissue as scar or healthy does not reflect the biological reality. In addition, the goal of quantitative measures of tissue composition will permit objective comparison between imaging techniques and their reproducibility. Future work will develop the experience of quantitative techniques of tissue characterisation and establish whether they bring additional value beyond standard LIE-CCT.
Cardiac CT imaging and potential utility in VT ablation with corresponding electrophysiological assessment. A and B Cardiac CT imaging demonstrating wall thinning in the basal to mid-infero- and anterolateral and apical lateral walls. C Polar plot showing the distribution of hypoperfusion defined on CCT according to a 17-segment model as a binary mask with the colour red indicating areas segmented as demonstrating reduced perfusion and labelled as a scar. D Left anterior oblique (LAO) view of bipolar voltage map during RV pacing and demonstrating a large bipolar voltage deficit in the inferolateral left ventricular endocardial wall. E Local activation time (LAT) map demonstrating late activation within bipolar deficit and corresponding late bipolar signals in this region. F Late potentials, representing electrophysiologic substrate for VT, identified on multi-polar mapping catheter at a position shown in E, within the region of imaging defined fibrosis (C) and low voltage region (D).
The precise localisation of myocardial substrate for VT with CCT has raised the possibility of using a purely image-guided VT ablation strategy, which would potentially widen access to VT ablation and increase procedural efficiency. Such an approach is being assessed in an ongoing randomised controlled trial [50].
CCT techniques continue to progress, and recent developments suggest the diagnostic utility of CCT for the assessment of substrate for ventricular arrhythmias will continue to increase. Advances in tissue characterisation may focus on the quantitative assessment of fibrosis through the assessment of ECV and the identification of epicardial adipose tissue and its relationship to arrhythmogenic substrate [51] as well as novel strategies for tissue characterisation including tissue heterogeneity which has been assessed in arrhythmogenic right ventricular cardiomyopathy [52]. The use of positron emission tomography (PET) CT may play a role in the identification of arrhythmias secondary to cardiac inflammation which may be best suited to management with anti-inflammatory medications as opposed to invasive ablation strategies [53•]. Photon-counting CT (PCCT) represents a technical advance in CT image acquisition, offering higher spatial resolution and better soft-tissue contrast, which may enable more precise tissue characterisation, as well as being less susceptible to blooming and beam-hardening artefact [54] which may be seen when imaging patients with ICD leads and areas of extensive calcification. In addition, PCCT may permit the use of novel contrast agents of particular relevance to patients with chronic kidney disease who cannot tolerate iodine-based contrast [55]. This imaging technique has now been used to acquire CCTA with encouraging results [56••]. Further studies will delineate the incremental benefit of higher-resolution imaging in defining the arrhythmogenic substrate in more detail. Finally, the detailed characterisation of arrhythmogenic substrate using CCT imaging represents an important research tool that has provided an independent method of tissue characterisation used to interpret the utility of novel electrophysiologic parameters [38, 41, 52, 57]. This represents a crucial role for advanced imaging modalities. Appreciation of the pathophysiologic differences between electrophysiologic substrate encountered in different disease processes has recently developed further [58]. As more data is collected about the specific characteristics of different disease processes, there is a crucial mutualistic relationship between clinical information, electrophysiologically observed parameters, and the structural substrate for which histological information is rarely available and therefore relies on non-invasive imaging to characterise.
Intra-cardiac echocardiography
Intra-cardiac echocardiography (ICE) is a tool that allows real-time cardiac imaging during a VT ablation procedure (Fig. 4). This has become an invaluable tool that has promoted the development of a clear understanding of anatomy relevant to contemporary electrophysiology procedures [59]. ICE may be used as a standalone tool with which to visualise cardiac (and extra-cardiac) structures or within the CartoSound module of the Carto3 (Biosense Webster, Diamond Bard, CA, USA) EAM system [60]. CartoSound permits the segmentation of structures in the same image space as the EAM data, which can provide both a 3D representation of the anatomic structures and their relationships as well as providing fiducial points which can be used to register pre-acquired cross-sectional imaging. There may be particular value in the use of ICE and the CartoSound segmentation module in the context of uncommon anatomical variants, which may be frequently encountered in the context of congenital heart disease [60].
Intra-cardiac echocardiography during VT ablation. A An intra-cardiac echocardiography showing the inferolateral wall of the left ventricle (corresponding electro-anatomic map in B) in a patient undergoing ablation for ventricular tachycardia occurring in the context of a mixed ischaemic and non-ischaemic cardiomyopathy. White arrows indicate sub-epicardial hyper-echoic areas consistent with a non-ischaemic scar that was also identified on cardiac magnetic resonance imaging. Green arrows indicate a sub-endocardial scar that was the result of an embolic myocardial infarction. The yellow star indicates the ablation catheter. B Electro-anatomic map of the left ventricle with colour scale according to bipolar voltage showing the inferolateral wall of the left ventricle. There is a relatively discrete bipolar voltage abnormality in the region of the sub-endocardial scar only, while the unipolar voltage abnormality extended more widely.
Within standard anatomic configurations, the value of ICE is most consistently evident when treating arrhythmias arising from the outflow tracts [61] and intra-cavity structures, including the LV papillary muscles [62, 63] and the right ventricular papillary muscle-moderator band complex (PM-MB) [64, 65]. In the outflow tract, safety considerations relate to the proximity of the coronary arteries to ablation targets. The ostia of the coronary arteries can be directly visualised with ICE and the proximity to an ablation target assessed. These structures may also be tagged or segmented in CartoSound such that their relationship to an ablation catheter or target can be demonstrated within the EAM system.
When arrhythmias arise from intra-cavity structures such as the papillary muscles (PM) of the left ventricle or the right ventricular PM-MB complex, assessment of contact with this structure may be greatly assisted by direct visualisation with ICE [65]. Common challenges encountered when ablating PM or MB-PM arrhythmias include achieving adequate contact and stability. This may be monitored during RF application and in the event of difficulties prompt consideration of alternative energy sources to overcome these challenges [62, 66, 67].
While not as sensitive for the comprehensive identification of substrate as CMR or CCT, ICE may be effective to identify fibrosis (Fig. 4A), which may appear as hyper-echoic tissue, and be identified in ischemic [68] and non-ischemic [69] cardiomyopathies, providing a real-time image of the substrate and its relationship to an ablation catheter.
The successful use of 4D echocardiography has been demonstrated in human subjects [70] and may be more widely available in the future. Artificial intelligence-driven automatic segmentation tools have been used widely in echocardiography [71] and when implemented in ICE systems may reduce the time needed for the acquisition of a 3D surface anatomy to guide ablation procedures. ICE is a versatile tool that represents the modality that offers the greatest anatomic detail in real-time during ablation procedures and as such has become an invaluable adjunct during catheter ablation of ventricular arrhythmias.
Image integration
In addition to the use of pre-procedural imaging for planning ablation procedures, the use of image integration software during ablation procedures is increasing. This may then permit operators to directly explore the imaging-identified substrate. This requires several steps in order to be successfully performed. Firstly, segmentation of the imaging is required. There are a number of tools available that may be used for this, and they typically use a combination of manual segmentation which may then be combined with artificial intelligence-based tools [72, 73]. An accurate segmentation of cross-sectional imaging is a necessary condition for the imaging to be helpfully integrated during an ablation procedure. Some tools which are commercially available include inHeart [74], ADAS3D [75], and CartoSeg [73]. In addition, there are open-source tools for manual or semi-automatic segmentation that can be used for this purpose [76]. Following segmentation of the imaging, a derived model can then be imported into a mapping system. Following import, this model must be registered with the EAM data. This again is a crucial step in order for the full value to be derived from the imaging and requires identification of fiducial landmarks which can be reliably identified in the segmentation and the EAM system. The use of ICE within the CartoSound module allows for the identification of fiducial landmarks (including coronary cusps, coronary vessels, and LV endocardial contours) within the EAM image space, which may therefore be used to register segmentations. In the absence of ultrasound, other strategies to identify fiducial landmarks may be used. While there are multiple steps required to successfully and accurately import an imaging-derived model into an EAM system, the ability to directly compare imaging and electrophysiological data during an ablation procedure permits a comprehensive assessment of data, which may be valuable in identifying an appropriate ablation target.
Conclusions
CMR, CCT, and ICE are complementary imaging modalities each of which have enhanced the operator’s ability to define the substrate for ventricular arrhythmias prior to and during ablation procedures. CMR offers the best soft-tissue contrast but is susceptible to artefact from CIED; CCT has the highest resolution and is relatively robust to artefact but is limited by lower soft-tissue contrast. All pre-procedural cross-sectional imaging depends on accurate registration if they are to be used for the purposes of direct image guidance during a procedure. Conformational changes in cardiac shape between the acquisition of imaging and the time of ablation present absolute limitations to the accuracy of any registration that is undertaken. To minimise further error, the use of fiducial landmarks from ICE is of significant value. ICE has the additional unique feature of offering real-time imaging during an ablation, which is helpful in patients who have not undergone pre-procedural imaging, in whom there have been significant conformational changes in the ventricle between imaging and ablation and when targeting intra-cavity structures. ICE may also identify the arrhythmic substrate, although it is less sensitive to the identification of fibrosis than CMR and CCT. Significant advances have been made in each modality. The introduction of wide-band imaging addressing the limitation of artefact from CIED in CMR, photon counting CCT to improving soft-tissue contrast, and the development of objective and quantitative measures of myocardial fibrosis through the use of ECV and the introduction of 4D ICE with automatic segmentation strategies represents important recent technological advances. A comprehensive ventricular substrate assessment may benefit from the use of each or all of these modalities in different circumstances.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126.
Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–520.
Ismail TF, Strugnell W, Coletti C, et al. Cardiac MR: from theory to practice. Front Cardiovasc Med. 2022;9.
Pereira RS, Prato FS, Wisenberg G, Sykes J. The determination of myocardial viability using Gd-DTPA in a canine model of acute myocardial ischemia and reperfusion. Magn Reson Med. 1996;36:684–93.
Saeed M, Wendland MF, Masui T, Higgins CB. Reperfused myocardial infarctions on T1- and susceptibility-enhanced MRI: evidence for loss of compartmentalization of contrast media. Magn Reson Med. 1994;31:31–9.
Kim RJ, Chen E-L, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318–26.
Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–9.
Schelbert EB, Hsu LY, Anderson SA, et al. Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging. 2010;3:743–52.
Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53.
Beek AM, Kühl HP, Bondarenko O, et al. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol. 2003;42:895–901.
Morton G, Schuster A, Perera D, Nagel E. Cardiac magnetic resonance imaging to guide complex revascularization in stable coronary artery disease. Eur Heart J. 2010;31:2209–15.
Dawson DK, Hawlisch K, Prescott G, et al. Prognostic role of CMR in patients presenting with ventricular arrhythmias. 2013.
Lintingre PF, Nivet H, Clément-Guinaudeau S, et al. High-resolution late gadolinium enhancement magnetic resonance for the diagnosis of myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging. 2020;13:1135–48.
Siontis KC, Kim HM, SharafDabbagh G, et al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2017;14:1487–93.
Disertori M, Rigoni M, Pace N, et al. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging. 2016;9:1046–55.
Levine YC, Matos J, Rosenberg MA, Manning WJ, Josephson ME, Buxton AE. Left ventricular sphericity independently predicts appropriate implantable cardioverter-defibrillator therapy. Heart Rhythm. 2016;13:490–7.
Sasaki T, Miller CF, Hansford R, et al. Myocardial structural associations with local electrograms: a study of postinfarct ventricular tachycardia pathophysiology and magnetic resonance-based noninvasive mapping. Circ Arrhythm Electrophysiol. 2012;5:1081–90.
Disertori M, Rigoni M, Pace N, et al. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction a meta-analysis. 2016.
Klem I, Klein M, Khan M, et al. Relationship of LVEF and myocardial scar to long-term mortality risk and mode of death in patients with nonischemic cardiomyopathy. Circulation. 2021;143:1343–58.
Piers SRD, Tao Q, De M, et al. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. 2014.
Fernández-Armenta J, Berruezo A, Andreu D, et al. Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: insights for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2013;6:528–37.
Andreu D, Berruezo A, Ortiz-Pérez JT, et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2011;4:674–83.
Andreu D, Penela D, Acosta J, et al. Cardiac magnetic resonance–aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm. 2017;14:1121–8.
Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. 2011.
Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 Tesla. Circulation. 2006;114:1277–84.
Klein-Wiele O, Garmer M, Urbien R, et al. Feasibility and safety of adenosine cardiovascular magnetic resonance in patients with MR conditional pacemaker systems at 1.5 Tesla. J Cardiovasc Magn Reson. 2015;17.
Rashid S, Rapacchi S, Vaseghi M, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology. 2014;270:269–74.
Orini M, Seraphim A, Graham A, et al. Detailed Assessment of low-voltage zones localization by cardiac MRI in patients with implantable devices. JACC Clin Electrophysiol. 2022;8:225–35.
Conte E, Mushtaq S, Carbucicchio C, et al. State of the art paper: cardiovascular CT for planning ventricular tachycardia ablation procedures. J Cardiovasc Comput Tomogr. 2021;15:394–402.
Yerly J, Becce F, Van Heeswijk RB, et al. In vitro optimization and comparison of CT angiography versus radial cardiovascular magnetic resonance for the quantification of cross-sectional areas and coronary endothelial function. J Cardiovasc Magn Reson. 2019;21.
Munnur RK, Cameron JD, Ko BS, Meredith IT, Wong DTL. Cardiac CT: atherosclerosis to acute coronary syndrome. Cardiovasc Diagn Ther. 2014;4:430–48.
Subramanian M, Ravilla VV, Yalagudri S, et al. CT-guided percutaneous epicardial access for ventricular tachycardia ablation: a proof-of-concept study. J Cardiovasc Electrophysiol. 2021;32:2665–72.
Conte E, Carbucicchio C, Catto V, et al. Live integration of comprehensive cardiac CT with electroanatomical mapping in patients with refractory ventricular tachycardia. J Cardiovasc Comput Tomogr. 2022;16:262–5.
Kowalewski C, Ascione C, Nuñez-Garcia M, et al. Advanced imaging integration for catheter ablation of ventricular tachycardia. Curr Cardiol Rep. 2023.
Rizvi A, Deaño RC, Bachman DP, Xiong G, Min JK, Truong QA. Analysis of ventricular function by CT. J Cardiovasc Comput Tomogr. 2015;9:1–12.
Busch JL, Alessio AM, Caldwell JH, et al. Myocardial hypo-enhancement on resting computed tomography angiography images accurately identifies myocardial hypoperfusion. J Cardiovasc Comput Tomogr. 2011;5:412–20.
Xu L, Khoshknab M, Berger RD, et al. Lipomatous metaplasia enables ventricular tachycardia by reducing current loss within the protected corridor. JACC Clin Electrophysiol. 2022;8:1274–85.
Yamashita S, Sacher F, Hooks DA, et al. Myocardial wall thinning predicts transmural substrate in patients with scar-related ventricular tachycardia. Heart Rhythm. 2017;14:155–63.
Takigawa M, Duchateau J, Sacher F, et al. Are wall thickness channels defined by computed tomography predictive of isthmuses of postinfarction ventricular tachycardia? Heart Rhythm. 2019;16:1661–8.
Ghannam M, Cochet H, Jais P, et al. Correlation between computer tomography-derived scar topography and critical ablation sites in postinfarction ventricular tachycardia. J Cardiovasc Electrophysiol. 2018;29:438–45.
Takigawa M, Martin R, Cheniti G, et al. Detailed comparison between the wall thickness and voltages in chronic myocardial infarction. J Cardiovasc Electrophysiol. 2019;30:195–204.
Maher TR, Freedman B, Locke AH, et al. Correlation between functional substrate mapping and cardiac computed tomography–derived wall thinning for ventricular tachycardia ablation. JACC Clin Electrophysiol. 2023. Published online July 2023. https://doi.org/10.1016/j.jacep.2023.05.018.
Schuleri KH, George RT, Lardo AC. Applications of cardiac multidetector CT beyond coronary angiography. Nat Rev Cardiol. 2009;6:699–710.
Palmisano A, Vignale D, Tadic M, et al. Myocardial late contrast enhancement CT in troponin positive acute chest pain syndrome. Radiology. 2022;302:545–53.
Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823–33.
• Chang S, Han K, Youn JC, et al. Utility of dual-energy CT-based monochromatic imaging in the assessment of myocardial delayed enhancement in patients with cardiomyopathy. Radiology. 2018;287:442–451. In this report, the benefits of dual-energy CT to identify delayed enhancement representing fibrosis in patients with cardiomyopathy were demonstrated, representing a significant advancement in the ability to identify substrate using cardiac CT.
Lee HJ, Im DJ, Youn JC, et al. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac ct in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology. 2016;280:49–57.
Hamdy A, Kitagawa K, Goto Y, et al. Comparison of the different imaging time points in delayed phase cardiac CT for myocardial scar assessment and extracellular volume fraction estimation in patients with old myocardial infarction. Int J Cardiovasc Imaging. 2019;35:917–26.
Yamada A, Kitagawa K, Nakamura S, et al. Quantification of extracellular volume fraction by cardiac computed tomography for noninvasive assessment of myocardial fibrosis in hemodialysis patients. Sci Rep. 2020;10.
Sacher F, Cochet H. Computed tomography-guided catheter ablation for ventricular tachycardia (InEurHeart). 2022.
Chan J, Thakur U, Tan S, et al. Inter-software and inter-scan variability in measurement of epicardial adipose tissue: a three-way comparison of a research-specific, a freeware and a coronary application software platform. Eur Radiol. 2023. Published online June 27, 2023. https://doi.org/10.1007/s00330-023-09878-5.
Venlet J, Tao Q, de Graaf MA, et al. RV tissue heterogeneity on CT: A Novel Tool to Identify the VT Substrate in ARVC. JACC Clin Electrophysiol. 2020;6:1073–85.
• Hayase J, Do DH, Liang JJ, et al. Detection of inflammation using cardiac positron emission tomography for evaluation of ventricular arrhythmias: an institutional experience. Heart Rhythm. 2022;19:2064–2072. This report demonstrates the value of PET-CT in detecting myocardial inflammation contributing to ventricular arrhythmias with important therapeutic implications surrounding the use of anti-inflammatory medications.
Taguchi K, Iwanczyk JS. Vision 20/20: single photon counting X-ray detectors in medical imaging. Med Phys. 2013;40: 100901.
Amato C. Novel Contrast Agents in Photon-Counting Computed Tomography.
•• Si-Mohamed SA, Boccalini S, Lacombe H, et al. Coronary CT angiography with photon-counting CT: first-in-human results. Radiology. 2022;303:303–313. In this study, the use of photon-counting cardiac computed tomography was demonstrated in humans for the first time, representing an advancement in the image quality beyond previous standard dual-energy CT-based imaging.
Whitaker J, Baum TE, Qian P, et al. Frequency domain analysis of endocardial electrograms for detection of nontransmural myocardial fibrosis in nonischemic cardiomyopathy. JACC Clin Electrophysiol. 2023. Published online January 2023. https://doi.org/10.1016/j.jacep.2022.11.019.
Glashan CA, Androulakis AFA, Tao Q, et al. Whole human heart histology to validate electroanatomical voltage mapping in patients with non-ischaemic cardiomyopathy and ventricular tachycardia. Eur Heart J. 2018;39:2867–75.
Pathak RK, Ariyarathna N, Garcia FC, Sanders P, Marchlinski FE. Catheter Ablation of idiopathic ventricular arrhythmias. Heart Lung Circ. 2019;28:102–9.
Kean AC, Gelehrter SK, Shetty I, Dick M, Bradley DJ. Experience with CartoSound for arrhythmia ablation in pediatric and congenital heart disease patients. J Interv Card Electrophysiol. 2010;29:139–45.
Van Herendael H, Zado ES, Haqqani H, et al. Catheter ablation of ventricular fibrillation: importance of left ventricular outflow tract and papillary muscle triggers. Heart Rhythm. 2014;11:566–73.
Kautzner J, Peichl P. Papillary muscle ventricular tachycardia or ectopy: diagnostics, catheter ablation and the role of intracardiac echocardiography. Arrhythm Electrophysiol Rev. 2019;8:65–9.
Yamada T, Yoshida N, Litovsky SH, Itoh T, Doppalapudi H, Kay GN. Idiopathic ventricular arrhythmias originating from the infundibular muscles prevalence, electrocardiographic and electrophysiological characteristics, and outcome of catheter ablation. Circ Arrhythm Electrophysiol. 2018;11.
Sadek MM, Benhayon D, Sureddi R, et al. Idiopathic ventricular arrhythmias originating from the moderator band: electrocardiographic characteristics and treatment by catheter ablation. Heart Rhythm. 2015;12:67–75.
Enriquez A, Supple GE, Marchlinski FE, Garcia FC. How to map and ablate papillary muscle ventricular arrhythmias. Heart Rhythm. 2017;14:1721–8.
Rivera S, De La M, Ricapito P, et al. Cryoablation for ventricular arrhythmias arising from the papillary muscles of the left ventricle guided by intracardiac echocardiography and image integration. 2015.
Whitaker J, Batnyam U, Kapur S, Sauer WH, Tedrow U. Safety and efficacy of cryoablation for right ventricular moderator band–papillary muscle complex ventricular arrhythmias. JACC Clin Electrophysiol. 2022. Published online April 2022. https://doi.org/10.1016/j.jacep.2022.03.011.
Qian PC, Tedrow UB. Intracardiac echocardiography to guide catheter ablation of ventricular arrhythmias in ischemic cardiomyopathy. Card Electrophysiol Clin. 2021;13:285–92.
Barrett C, Tzou WS. Utility of intracardiac echocardiography for guiding ablation of ventricular tachycardia in nonischemic cardiomyopathy. Card Electrophysiol Clin. 2021;13:337–43.
Katie Arnold. NuVera announces successful first-in-human use of NuVisionTM ICE catheter. 2020. https://www.prnewswire.com/news-releases/nuvera-announces-successful-first-in-human-use-of-nuvision-ice-catheter-301087888.html.
Kim T, Hedayat M, Vaitkus VV, Belohlavek M, Krishnamurthy V, Borazjani I. Automatic segmentation of the left ventricle in echocardiographic images using convolutional neural networks. Quant Imaging Med Surg. 2021;11:1763–81.
Yao Z, Xie W, Zhang J, et al. Graph matching and deep neural networks based whole heart and great vessel segmentation in congenital heart disease. Sci Rep. 2023;13.
Imanli H, Bhatty S, Jeudy J, et al. Validation of a novel CARTOSEG™ segmentation module software for contrast-enhanced computed tomography-guided radiofrequency ablation in patients with atrial fibrillation. PACE Pacing Clin Electrophysiol. 2017;40:1206–12.
Ene E, Halbfaß P, Nentwich K, et al. Optimal cut-off value for endocardial bipolar voltage mapping using a multipoint mapping catheter to characterize the scar regions described in cardio—CT with myocardial thinning. J Cardiovasc Electrophysiol. 2022;33:2174–80.
Jáuregui B, Soto-Iglesias D, Penela D, et al. Cardiovascular magnetic resonance determinants of ventricular arrhythmic events after myocardial infarction. Europace. 2022;24:938–47.
Solís-Lemus JA, Baptiste T, Barrows R, et al. Evaluation of an open-source pipeline to create patient-specific left atrial models: a reproducibility study. Comput Biol Med. 2023;162.
Author information
Authors and Affiliations
Contributions
JW wrote the first draft of the manuscript. TI made critical revisions to the MRI section of the manuscript. RR made critical revisions to the CT section of the manuscript. MW/PZ reviewed the article and made substantive changes throughout. All authors contributed to the subsequent revisions of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Matthew Wright reports personal fees from Philips and Biosense Webster outside the submitted work. Paul C. Zei reports Biosense Webster—consultant and research support and Abbott—consultant and research support. John Whitaker declares that he has no conflict of interest. Ronak Rajani declares that he has no conflict of interest. Tevfik F. Ismail declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Whitaker, J., Rajani, R., Ismail, T.F. et al. The Use of Pre- and Peri-Procedural Imaging During VT Ablation. Curr Treat Options Cardio Med 26, 13–28 (2024). https://doi.org/10.1007/s11936-023-01031-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-023-01031-1