Abstract
Purpose of review
To discuss invasive cardiovascular procedures as they relate to onco-cardiology, as well as recent additions to anti-cancer therapies and their subsequent effect on cardiovascular toxicity.
Recent findings
The development of immune checkpoint inhibitors and chimeric antigen receptor T cell therapy has been linked to cardiotoxicity and represents an emerging area of concern. Recent advances in transcatheter valve replacement have shown benefits compared with surgical management regardless of malignancy type, stage, or treatment.
Summary
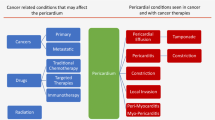
With the increasing use of immunotherapy and increasing recognition of cardiotoxicity, there is a need for identifying mortality-improving strategies. The use of a transcatheter approach for aortic valve replacement looks to be a safer alternative when compared with surgical replacement despite the presence of cancer. Pericardial disease is frequent in the cancer population and pericardiocentesis represents a valid option for the treatment of significant pericardial effusions. Endomyocardial biopsy is performed for various indications in the cancer population and is the gold standard for diagnosing myocarditis and infiltrative diseases.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Miller KD, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2019;69(5):363–85.
Zaorsky NG, et al. Causes of death among cancer patients. Ann Oncol : official journal of the European Society for Medical Oncology. 2017;28(2):400–7.
Potts JE, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J. 2018;40(22):1790–800.
Kitsis Richard N, Jaime AR, Lavandero S. Heart disease and cancer. Circulation. 2018;138(7):692–5.
Oren O, Herrmann J. Arterial events in cancer patients-the case of acute coronary thrombosis. J Thorac Dis. 2018;10(Suppl 35):S4367–s4385.
Iliescu CA, et al. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologıa intervencionista). Catheter Cardiovasc Interv. 2016;87(5):E202–23.
•• Herrmann J, et al. Vascular toxicities of cancer therapies. Circulation. 2016;133(13):1272–8. The most important paper on the toxicity of cancer therapy.
Lopez-Mattei J, Kim P, Iliescu C. Editorial Commentary: Update on cardio-oncology: novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc Med. 2019;29(1):40.
• Marmagkiolis K, et al. Radiation toxicity to the cardiovascular system. Curr Oncol Rep. 2016;18(3):1. First experience of MitraClip in cancer patients.
Zarifa A, et al. Cardiac toxicities of anticancer treatments: chemotherapy, targeted therapy and immunotherapy. Curr Opin Cardiol. 2019;34(4):441–50.
Sorrentino MF, et al. 5-Fluorouracil induced cardiotoxicity: review of the literature. Cardiol J. 2012;19(5):453–8.
Das SK, Das AK, William M. 5-Fluorouracil-induced acute coronary syndrome. Med J Aust. 2019;211(6):255–257.e1.
Felix-Oliveira A, et al. Acute coronary syndrome in the oncology patient: an avoidable event? Rev Port Cardiol. 2018;37(9):791.e1–4.
Chong JH, Ghosh AK. Coronary artery vasospasm induced by 5-fluorouracil: proposed mechanisms, existing management options and future directions. Interv Cardiol. 2019;14(2):89–94.
Mosseri M, et al. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993;53(13):3028–33.
Ben-Yakov M, et al. Prinzmetal angina (Coronary vasospasm) associated with 5-fluorouracil chemotherapy. Am J Emerg Med. 2017;35(7):1038.e3–5.
Ray JC, et al. A case of 5-fluorouracil-induced cardiac arrest. J Emerg Med. 2016;50(1):e1–6.
Ma WW, et al. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer. 2017;123(2):345–56.
Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191–202.
Redman JM, et al. Successful 5-fluorouracil (5-FU) infusion re-challenge in a metastatic colorectal cancer patient with coronary artery disease who experienced symptoms consistent with coronary vasospasm during first 5-FU infusion. J Gastrointest Oncol. 2019;10(5):1010–4.
Somov P, et al. Spontaneous coronary artery dissection during cisplatin and capecitabine therapy. Ann Med Surg (Lond). 2019;45:1–5.
Ozturk MA, et al. Takotsubo syndrome: an underdiagnosed complication of 5-fluorouracil mimicking acute myocardial infarction. Blood Coagul Fibrinolysis. 2013;24(1):90–4.
Zhao D, et al. Atrial fibrillation following treatment with paclitaxel: a case report. Biomed Rep. 2018;9(6):540–4.
Rowinsky EK, et al. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9(9):1704–12.
Shah K, et al. Acute non-ST elevation myocardial infarction following paclitaxel administration for ovarian carcinoma: a case report and review of literature. J Cancer Res Ther. 2012;8(3):442–4.
Schrader C, et al. Symptoms and signs of an acute myocardial ischemia caused by chemotherapy with paclitaxel (Taxol) in a patient with metastatic ovarian carcinoma. Eur J Med Res. 2005;10(11):498–501.
Osman M, Elkady M. A prospective study to evaluate the effect of paclitaxel on cardiac ejection fraction. Breast Care (Basel). 2017;12(4):255–9.
Berliner S, et al. Acute coronary events following cisplatin-based chemotherapy. Cancer Investig. 1990;8(6):583–6.
Karabay KO, Yildiz O, Aytekin V. Multiple coronary thrombi with cisplatin. J Invasive Cardiol. 2014;26(2):E18–20.
Hanchate LP, Sharma SR, Madyalkar S. Cisplatin induced acute myocardial infarction and dyslipidemia. J Clin Diagn Res : JCDR. 2017;11(6):OD05–7.
Jafri M, Protheroe A. Cisplatin-associated thrombosis. Anti-Cancer Drugs. 2008;19(9):927–9.
Meinardi MT, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. 2000;18(8):1725–32.
Gietema JA, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet. 2000;355(9209):1075–6.
Huddart RA, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21(8):1513–23.
van den Belt-Dusebout AW, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2006;24(3):467–75.
Haugnes HS, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010;28(30):4649–57.
Feldman DR, Schaffer WL, Steingart RM. Late cardiovascular toxicity following chemotherapy for germ cell tumors. J Natl Compr Cancer Netw. 2012;10(4):537–44.
Bassareo PP, et al. Multimodality imaging diagnosis of multiple ventricular thrombosis and massive stroke after gemcitabine and cisplatin chemotherapy for urothelial cancer. J Cardiovasc Echogr. 2019;29(2):71–4.
Seidman A, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–21.
Crone SA, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–65.
Cardinale D, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–6.
Vaklavas C, et al. Anti-vascular endothelial growth factor therapies and cardiovascular toxicity: what are the important clinical markers to target? Oncologist. 2010;15(2):130–41.
Ranpura V, et al. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens. 2010;23(5):460–8.
Ma W, et al. Cardiotoxicity of sorafenib is mediated through elevation of ROS level and CaMKII activity and dysregulation of calcium homoeostasis. Basic Clin Pharmacol Toxicol. 2019;126:166–80.
Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018;2:13.
Park JG, et al. Long-term follow-up of complete remission of advanced hepatocellular carcinoma following sorafenib therapy: a case report. Oncol Lett. 2017;14(4):4853–6.
Sudasena D, et al. Fulminant vascular and cardiac toxicity associated with tyrosine kinase inhibitor sorafenib. Cardiovasc Toxicol. 2019;19(4):382–7.
Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48(1):9–17.
Wu S, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9(2):117–23.
Ranpura V, et al. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49(3):287–97.
Chu TF, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–9.
Herrmann J, Lerman A. An update on cardio-oncology. Trends Cardiovasc Med. 2014;24(7):285–95.
Mozolevska V, et al. Role of renin-angiotensin system antagonists in the prevention of bevacizumab- and sunitinib-mediated cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2019;316(3):H446–h458.
Levato L, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institution study. Eur J Haematol. 2013;90(6):531–2.
Li L, et al. Acute ischemic intestinal necrosis as a rare side effect of nilotinib. Niger J Clin Pract. 2019;22(1):131–3.
Latifi Y, et al. Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood. 2019;133(14):1597–606.
Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33(35):4210–8.
Barber MC, Mauro MJ, Moslehi J. Cardiovascular care of patients with chronic myeloid leukemia (CML) on tyrosine kinase inhibitor (TKI) therapy. Hematol Am Soc Hematol Educ Program. 2017;2017(1):110–4.
Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2012;34(15):1102–11.
Gottdiener JS, et al. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141(6):758–63.
Cesarman-Maus G, Braggio E, Fonseca R. Thrombosis in multiple myeloma (MM). Hematology. 2012;17(sup1):s177–80.
Lyon AR, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–58.
Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–7.
Nishino M, et al. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373(3):288–90.
Hsu CY, Su YW, Chen SC. Sick sinus syndrome associated with anti-programmed cell death-1. J Immunother Cancer. 2018;6(1):72.
Reddy N, et al. Progressive and reversible conduction disease with checkpoint inhibitors. Can J Cardiol. 2017;33(10):1335.e13–5.
Salem JE, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89.
Ederhy S, et al. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc Imaging. 2018;11(8):1187–90.
Lindner AK, et al., Rare, but severe: vasculitis and checkpoint inhibitors. Eur Urol Focus, 2019.
Franco F, et al. Nivolumab-associated digital small-vessel vasculitis in a patient with an advanced renal cell carcinoma. Immunotherapy. 2019;11(5):379–84.
Wang J, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–52.
Lucas JA, et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181(4):2513–21.
Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22.
Okazaki T, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–83.
Ferreira M, et al. Coronary toxicities of anti-PD-1 and anti-PD-L1 immunotherapies: a case report and review of the literature and international registries. Target Oncol. 2018;13(4):509–15.
Ganatra S, neilan tg. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23(8):879–86.
Mahmood SS, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64.
Johnson DB, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55.
Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Zhang L, et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Treat Options Cardiovasc Med. 2019;21(7):32.
Heinzerling L, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50.
Gramatyka M, Skorupa A, Sokol M. Nuclear magnetic resonance spectroscopy reveals metabolic changes in living cardiomyocytes after low doses of ionizing radiation. Acta Biochim Pol. 2018;65(2):309–18.
Han X, Zhou Y, Liu W. Precision cardio-oncology: understanding the cardiotoxicity of cancer therapy. NPJ Precis Oncol. 2017;1(1):31.
Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98.
Dess RT, et al. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol. 2017;35(13):1395–402.
McEniery PT, et al. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol. 1987;60(13):1020–4.
Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27(8):766–73.
Virmani R, et al. Comparative pathology: radiation-induced coronary artery disease in man and animals. Semin Interv Cardiol. 1998;3(3–4):163–72.
Orzan F, et al. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J. 1993;69(6):496–500.
Zamorano JL, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801.
Lenihan DJ, Cuculich P. Cardioprotection during therapeutic radiation treatment. Circ Heart Fail. 2018;11(8):e005294.
Chun SG, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56–62.
Chang JY, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1087–96.
Shah C, et al. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol. 2014;112(1):9–16.
Verma V, Shah C, Mehta MP. Clinical outcomes and toxicity of proton radiotherapy for breast cancer. Clin Breast Cancer. 2016;16(3):145–54.
Kammerer E, et al. Proton therapy for locally advanced breast cancer: a systematic review of the literature. Cancer Treat Rev. 2018;63:19–27.
Taylor CW, et al. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93(4):845–53.
Chang HM, et al. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. 2017;70(20):2536–51.
Gujral DM, Lloyd G, Bhattacharyya S. Radiation-induced valvular heart disease. Heart. 2016;102(4):269–76.
Faggiano P, et al. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision-making process. Int J Cardiol. 2012;159(2):94–9.
Nielsen HH. Transcatheter aortic valve implantation. Dan Med J. 2012;59(12):B4556.
Leon MB, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–607.
Popma JJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–15.
Mack MJ, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–705.
Watanabe Y, et al. Comparison of results of transcatheter aortic valve implantation in patients with versus without active cancer. Am J Cardiol. 2016;118(4):572–7.
Schechter M, et al. An update on the management and outcomes of cancer patients with severe aortic stenosis. Catheter Cardiovasc Interv. 2018;94(3):438–45 0(0).
Landes U, et al. Transcatheter aortic valve replacement in oncology patients with severe aortic stenosis. JACC Cardiovasc Interv. 2019;12(1):78–86.
Mangner N, et al. Impact of active cancer disease on the outcome of patients undergoing transcatheter aortic valve replacement. J Interv Cardiol. 2018;31(2):188–96.
Berkovitch A, et al. Favorable short-term and long-term outcomes among patients with prior history of malignancy undergoing transcatheter aortic valve implantation. J Invasive Cardiol. 2018;30(3):105–9.
Kogoj P, Devjak R, Bunc M. Balloon aortic valvuloplasty (BAV) as a bridge to aortic valve replacement in cancer patients who require urgent non-cardiac surgery. Radiol Oncol. 2014;48(1):62–6.
Balanescu SM, et al. The onco-cardiologist dilemma: to implant, to defer, or to avoid transcatheter aortic valve replacement in cancer patients with aortic stenosis? Curr Cardiol Rep. 2019;21(8):83.
• Marmagkiolis K, et al. Clinical outcomes of percutaneous mitral valve repair with MitraClip for the management of functional mitral regurgitation. Catheter Cardiovasc Interv. 2019;94(6):820–6. The most complete manuscript explaining the the mechanisms of radiation-induced toxicity.
Cerillo AG, et al. Transapical transcatheter valve-in-valve implantation for failed mitral bioprostheses: gradient, symptoms, and functional status in 18 high-risk patients up to 5 years. Ann Thorac Surg. 2016;102(4):1289–95.
Drury JH, Labovitz AJ, Miller LW. Echocardiographic guidance for endomyocardial biopsy. Echocardiography. 1997;14(5):469–74.
Balanescu DV, et al. The 1, 2, 3, 4 of carcinoid heart disease: comprehensive cardiovascular imaging is the mainstay of complex surgical treatment. Oncol Lett. 2019;17(5):4126–32.
Kesarwani M, et al. First-in-human transcatheter pulmonic valve implantation through a tricuspid valve bioprosthesis to treat native pulmonary valve regurgitation caused by carcinoid syndrome. JACC Cardiovasc Interv. 2015;8(10):e161–3.
Khan JN, et al. Transcatheter pulmonary and tricuspid valve-in-valve replacement for bioprosthesis degeneration in carcinoid heart disease. Eur Heart J Cardiovasc Imaging. 2016;17(1):114.
Loyalka P, et al. Transcatheter pulmonary valve replacement in a carcinoid heart. Tex Heart Inst J. 2016;43(4):341–4.
Conradi L, et al. Carcinoid heart valve disease: transcatheter pulmonary valve-in-valve implantation in failing biological xenografts. J Heart Valve Dis. 2015;24(1):110–4.
Whitlock MC, et al. Cancer and its association with the development of coronary artery calcification: an assessment from the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2015;4(11):e002533.
Navi BB, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70(8):926–38.
Fuster V, et al. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46(6):937–54.
Meyer CC, et al. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy. 1997;17(4):729–36.
Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47.
Czaykowski PM, Moore MJ, Tannock IF. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 1998;160(6 Pt 1):2021–4.
Caldemeyer L, et al. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Hematol Malig Rep. 2016;11(2):71–9.
Schutz FA, et al. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22(6):1404–12.
Torres M, Moayedi S. Evaluation of the acutely dyspneic elderly patient. Clin Geriatr Med. 2007;23(2):307–25.
Yusuf SW, et al. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35(7):443–50.
Mamas MA, et al. Prevalence and impact of co-morbidity burden as defined by the Charlson co-morbidity index on 30-day and 1- and 5-year outcomes after coronary stent implantation (from the Nobori-2 Study). Am J Cardiol. 2015;116(3):364–71.
Guddati AK, Joy PS, Kumar G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J Cancer Res Clin Oncol. 2016;142(2):471–9.
Levine GN, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–55.
Iliescu CA, et al. “Bringing on the light” in a complex clinical scenario: optical coherence tomography–guided discontinuation of antiplatelet therapy in cancer patients with coronary artery disease (PROTECT-OCT registry). Am Heart J. 2017;194:83–91.
Lee DH, de la torre hernandez jm. The newest generation of drug-eluting stents and beyond. Eur Cardiol. 2018;13(1):54–9.
Akashi YJ, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118(25):2754–62.
Lee S, et al. Stress-induced cardiomyopathy during pulmonary resection (Takotsubo syndrome) - a case report. Korean J Thorac Cardiovasc Surg. 2011;44(4):294–7.
van de Donk NW, et al. Takotsubo cardiomyopathy following radioiodine therapy for toxic multinodular goitre. Neth J Med. 2009;67(10):350–2.
Sharkey SW, et al. Natural history and expansive clinical profile of stress (Tako-Tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55(4):333–41.
Komamura K, et al. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6(7):602–9.
Giza DE, et al. Stress-Induced cardiomyopathy in cancer patients. Am J Cardiol. 2017;120(12):2284–8.
Munoz E, et al. Takotsubo stress cardiomyopathy: “good news” in cancer patients? J Am Coll Cardiol. 2016;68(10):1143–4.
Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408–17.
Boden WE, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16.
De Bruyne B, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208–17.
Elting LS, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19(4):1137–46.
Iliescu C, et al. Safety of diagnostic and therapeutic cardiac catheterization in cancer patients with acute coronary syndrome and chronic thrombocytopenia. Am J Cardiol. 2018;122(9):1465–70.
Iliescu C, et al. Safety of diagnostic and therapeutic cardiac catheterization in cancer patients with acute coronary syndrome and chronic thrombocytopenia. Am J Cardiol. 2018;122(9):1465–70.
Iliescu C, Durand J-B, Kroll M. Cardiovascular interventions in thrombocytopenic cancer patients. Tex Heart Inst J. 2011;38(3):259–60.
Yeh ET, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–31.
Jaworski C, et al. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61(23):2319–28.
Williams PD, et al. Right and left heart catheterization via an antecubital fossa vein and the radial artery--a prospective study. J Invasive Cardiol. 2014;26(12):669–73.
Singh V, et al. Comparison of utilization trends, indications, and complications of endomyocardial biopsy in native versus donor hearts (from the Nationwide Inpatient Sample 2002 to 2014). Am J Cardiol. 2018;121(3):356–63.
Cooper Leslie T, et al. The role of endomyocardial biopsy in the management of cardiovascular disease. Circulation. 2007;116(19):2216–33.
Ishibashi-Ueda H, et al. Significance and value of endomyocardial biopsy based on our own experience. Circ J. 2017;81(4):417–26.
Francis R, Lewis C. Myocardial biopsy: techniques and indications. Heart. 2018;104(11):950–8.
Anderson JL, Marshall HW. The femoral venous approach to endomyocardial biopsy: comparison with internal jugular and transarterial approaches. Am J Cardiol. 1984;53(6):833–7.
Ardehali H, Kasper EK, Baughman KL. Diagnostic approach to the patient with cardiomyopathy: whom to biopsy. Am Heart J. 2005;149(1):7–12.
Brooksby IA, et al. Left-ventricular endomyocardial biopsy. Lancet. 1974;2(7891):1222–5.
Escher F, et al. Analysis of endomyocardial biopsies in suspected myocarditis—Diagnostic value of left versus right ventricular biopsy. Int J Cardiol. 2014;177(1):76–8.
Miller LW, et al. Echocardiography-guided endomyocardial biopsy. A 5-year experience. Circulation. 1988;78(5 Pt 2):Iii99–102.
From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86(11):1095–102.
Kreher SK, et al. Frequent occurrence of occult pulmonary embolism from venous sheaths during endomyocardial biopsy. J Am Coll Cardiol. 1992;19(3):581–5.
Sandhu JS, et al. Coronary artery fistula in the heart transplant patient. A potential complication of endomyocardial biopsy. Circulation. 1989;79(2):350–6.
Wong RC, et al. Tricuspid regurgitation after cardiac transplantation: an old problem revisited. J Heart Lung Transplant. 2008;27(3):247–52.
Caforio AL, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48 2648a-2648d.
Kindermann I, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118(6):639–48.
Baccouche H, et al. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J. 2009;30(23):2869–79.
Veinot JP. Endomyocardial biopsy--when and how? Cardiovasc Pathol. 2011;20(5):291–6.
Leone O, et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21(4):245–74.
Caforio ALP, et al. How to improve therapy in myocarditis: role of cardiovascular magnetic resonance and of endomyocardial biopsy. Eur Heart J Suppl : journal of the European Society of Cardiology. 2019;21(Suppl B):B19–22.
Kim JS, et al. Cardiac sarcoidosis. Am Heart J. 2009;157(1):9–21.
Kandolin R, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624–32.
Uemura A, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138(2 Pt 1):299–302.
Maleszewski JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol. 2015;24(6):343–50.
Bisel HF, Wroblewski F, Ladue JS. Incidence and clinical manifestations of cardiac metastases. J Am Med Assoc. 1953;153(8):712–5.
Hoffmeier A, et al. Cardiac tumors--diagnosis and surgical treatment. Deutsches Arzteblatt Int. 2014;111(12):205–11.
Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261–2.
Palaskas N, et al. Evaluation and management of cardiac tumors. Curr Treat Options Cardiovasc Med. 2018;20(4):29.
Donisan T, et al. In search of a less invasive approach to cardiac tumor diagnosis: multimodality imaging assessment and biopsy. JACC Cardiovasc Imaging. 2018;11(8):1191–5.
Gilkeson RC, Chiles C. MR evaluation of cardiac and pericardial malignancy. Magn Reson Imaging Clin N Am. 2003;11(1):173–86 viii.
Hoey ET, et al. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009;64(12):1214–30.
Chan KL, et al. Diagnosis of left atrial sarcoma by transvenous endocardial biopsy. Can J Cardiol. 2001;17(2):206–8.
Ghosh AK, et al. Pericardial disease in cancer patients. Curr Treat Options Cardiovasc Med. 2018;20(7):60.
Imazio M, et al. Good prognosis for pericarditis with and without myocardial involvement: results from a multicenter, prospective cohort study. Circulation. 2013;128(1):42–9.
Sogaard KK, et al. Pericarditis as a marker of occult cancer and a prognostic factor for cancer mortality. Circulation. 2017;136(11):996–1006.
Kyto V, Sipila J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130(18):1601–6.
Pawlak Cieslik A, et al. Diagnosis of malignant pericarditis: a single centre experience. Kardiol Pol. 2012;70(11):1147–53.
Kim SH, et al. Clinical characteristics of malignant pericardial effusion associated with recurrence and survival. Cancer Res Treat. 2010;42(4):210–6.
Imazio M, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916–28.
Ben-Horin S, et al. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine (Baltimore). 2006;85(1):49–53.
Gagliardi G, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S77–85.
Wang K, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–94.
Bristow MR, et al. Early anthracycline cardiotoxicity. Am J Med. 1978;65(5):823–32.
van Rijssel RH, et al. A case of ATRA-induced isolated myocarditis in the absence of circulating malignant cells: demonstration of the t(15;17) translocation in the inflammatory infiltrate by in situ hybridisation. Leuk Res. 2010;34(7):e142–4.
Cham WC, et al. Radiation therapy of cardiac and pericardial metastases. Radiology. 1975;114(3):701–4.
Posner MR, Cohen GI, Skarin AT. Pericardial disease in patients with cancer. The differentiation of malignant from idiopathic and radiation-induced pericarditis. Am J Med. 1981;71(3):407–13.
Buck M, et al. Pericardial effusion in women with breast cancer. Cancer. 1987;60(2):263–9.
Restrepo CS, et al. Primary pericardial tumors. Radiographics. 2013;33(6):1613–30.
Tsang TS, et al. Echocardiographically guided pericardiocentesis: evolution and state-of-the-art technique. Mayo Clin Proc. 1998;73(7):647–52.
Maisch B, et al. Percutaneous therapy in pericardial diseases. Cardiol Clin. 2017;35(4):567–88.
Lekhakul A, et al. Safety and outcome of percutaneous drainage of pericardial effusions in patients with cancer. Am J Cardiol. 2018;122(6):1091–4.
Vilela EM, et al. Computed tomography-guided pericardiocentesis: a systematic review concerning contemporary evidence and future perspectives. Ther Adv Cardiovasc Dis. 2018;12(11):299–307.
Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. Jama. 1994;272(1):59–64.
El Haddad D, et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion. J Am Coll Cardiol. 2015;66(10):1119–28.
Maisch B, et al. Neoplastic pericardial effusion. Efficacy and safety of intrapericardial treatment with cisplatin. Eur Heart J. 2002;23(20):1625–31.
Iliescu C, et al. Echocardiography and fluoroscopy-guided pericardiocentesis for cancer patients with cardiac tamponade and thrombocytopenia. J Am Coll Cardiol. 2016;68(7):771–3.
Al-Hawwas M, et al. Acute coronary syndrome management in cancer patients. Curr Oncol Rep. 2018;20(10):78.
Rafique AM, et al. Frequency of recurrence of pericardial tamponade in patients with extended versus nonextended pericardial catheter drainage. Am J Cardiol. 2011;108(12):1820–5.
Adler Y, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921–64.
Bhardwaj R, et al. Evaluation of safety and feasibility of percutaneous balloon pericardiotomy in hemodynamically significant pericardial effusion (review of 10-years experience in single center). J Interv Cardiol. 2015;28(5):409–14.
Swanson N, et al. Primary percutaneous balloon pericardiotomy for malignant pericardial effusion. Catheter Cardiovasc Interv. 2008;71(4):504–7.
Ziskind AA, et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: description of technique and report of the first 50 cases. J Am Coll Cardiol. 1993;21(1):1–5.
Kunitoh H, et al. A randomised trial of intrapericardial bleomycin for malignant pericardial effusion with lung cancer (JCOG9811). Br J Cancer. 2009;100(3):464–9.
Zamorano JL, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801.
Felker GM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077–84.
Oliveira GH, et al. Characteristics and survival of patients with chemotherapy-induced cardiomyopathy undergoing heart transplantation. J Heart Lung Transplant. 2012;31(8):805–10.
Bianco CM, Al-Kindi SG, Oliveira GH. Advanced heart failure therapies for cancer therapeutics-related cardiac dysfunction. Heart Fail Clin. 2017;13(2):327–36.
Russo AM, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61(12):1318–68.
Rickard J, et al. Usefulness of cardiac resynchronization therapy in patients with adriamycin-induced cardiomyopathy. Am J Cardiol. 2010;105(4):522–6.
Ajijola OA, et al. Usefulness of cardiac resynchronization therapy in the management of doxorubicin-induced cardiomyopathy. Am J Cardiol. 2008;101(9):1371–2.
Fadol, A.P., E. Mouhayar, and C.C. Reyes-Gibby, The use of cardiac resynchronization therapy in cancer patients with heart failure. J Clin Exp Res Cardiol, 2017. 3(1).
Kirklin JK, et al. Long-term mechanical circulatory support (destination therapy) on track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144(3):584–603.
Slaughter MS, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–51.
Deo SV, Al-Kindi SG, Oliveira GH. Management of advanced heart failure due to cancer therapy: the present role of mechanical circulatory support and cardiac transplantation. Curr Treat Options Cardiovasc Med. 2015;17(6):388.
Abraham WT, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–66.
Adamson PB, et al. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ Heart Fail. 2016;9(6):e002600.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bala Pushparaji declares that there is no conflict of interest. Konstantinos Marmagkiolis declares that there is no conflict of interest. Cameron Miller declares that there is no conflict of interest. Moez K. Aziz declares that there is no conflict of interest. Dinu V. Balanescu declares that there is no conflict of interest. Teodora Donisan declares that there is no conflict of interest. Nicolas Palaskas declares that there is no conflict of interest. Peter Kim declares that there is no conflict of interest. Juan Lopez-Mattei declares that there is no conflict of interest. Mehmet Cilingiroglu declares that there is no conflict of interest. Saamir A. Hassan declares that there is no conflict of interest. Cezar A. Iliescu declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Coronary Artery Disease
Rights and permissions
About this article
Cite this article
Pushparaji, B., Marmagkiolis, K., Miller, C.K. et al. State-of-the-art Review: Interventional Onco-Cardiology. Curr Treat Options Cardio Med 22, 11 (2020). https://doi.org/10.1007/s11936-020-00809-x
Published:
DOI: https://doi.org/10.1007/s11936-020-00809-x