Abstract
Purpose of Review
A constellation of persistent adverse effects—collectively termed post-finasteride syndrome (PFS)—after 5α-reductase inhibitor treatment for benign prostatic hyperplasia (BPH) and androgenic alopecia (AGA) has recently been described. The severity of these sexual, physical, neurological, and psychiatric side effects raises important concerns regarding the treatment of these conditions, especially given the prevalence of indications for these medications. Here we review the literature exploring the symptoms, proposed mechanisms, and potential disease modulating factors for PFS.
Recent Findings
While the persistent sexual side effects associated with PFS are well documented, research on the physical, neurological, and psychiatric adverse effects is much less ubiquitous. Though the mechanisms leading to PFS have been proposed, a clear treatment plan for these patients has not been established. In the treatment of BPH and AGA with 5α-reductase inhibitors, the risks of PFS should be considered.
Summary
The occurrence of persistent adverse sexual, physical, neurological, and psychiatric side effects after 5α-reductase inhibitor is well supported by the existing data. While additional studies are needed to better evaluate the role of 5α-reductase inhibitors in the manifestation of the symptoms of PFS, the risks of PFS should be critically evaluated when treating patients with BPH or AGA.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Foundation P-FS. Overview. 2013. http://www.pfsfoundation.org/post-finasteride-syndrome-overview/.
Research CfDEa. Approval package for application number NDA 20-788/S-020/S-021/S-023. 2011.
Gupta AK, Sharma N, Shukla P. Atypical post-finasteride syndrome: a pharmacological riddle. Indian J Pharmacol. 2016;48(3):316–7. https://doi.org/10.4103/0253-7613.182898.
Reddy GK, Sartor O. Finasteride, a selective 5-alpha-reductase inhibitor, in the prevention and treatment of human prostate cancer. Clin Prostate Cancer. 2004;2(4):206–8. https://doi.org/10.1016/S1540-0352(11)70045-2.
Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179–84. https://doi.org/10.1210/jc.2003-030330.
Kiguradze T, Temps WH, Yarnold PR, Cashy J, Brannigan RE, Nardone B, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:e3020. https://doi.org/10.7717/peerj.3020.
Bartsch G, Rittmaster R, Klocker H. Dihydrotestosterone and the concept of 5α-reductase inhibition in human benign prostatic hyperplasia. World J Urol. 2002;19(6):413–25. https://doi.org/10.1007/s00345-002-0248-5.
Zito PM, Hin N. Finasteride in androgenic alopecia. J Dermatol Nurses’ Assoc. 2017;9(1):43–5. https://doi.org/10.1097/JDN.0000000000000279.
Hoffmann R, Happle R. Current understanding of androgenetic alopecia. Part II: clinical aspects and treatment. Eur J Dermatol: EJD. 2000;10(5):410–7.
Lu S-H, Chen C-S. Natural history and epidemiology of benign prostatic hyperplasia. Formosan J Surg. 2014;47(6):207–10. https://doi.org/10.1016/j.fjs.2014.10.001.
Basaria S, Jasuja R, Huang G, Wharton W, Pan H, Pencina K, et al. Characteristics of men who report persistent sexual symptoms after finasteride use for hair loss. J Clin Endocrinol Metab. 2016;101(12):4669–80. https://doi.org/10.1210/jc.2016-2726. This study compares PFS patient body composition, fMRI responses, sexual function, mood, cognition, and hormonal levels to those of asymptomatic finasteride users and healthy controls to investigate the pathophysiologic mechanism of PFS
Liu L, Zhao S, Li F, Li E, Kang R, Luo L, et al. Effect of 5alpha-reductase inhibitors on sexual function: a meta-analysis and systematic review of randomized controlled trials. J Sex Med. 2016;13(9):1297–310. https://doi.org/10.1016/j.jsxm.2016.07.006. This comprehensive meta-analysis examines changes in sexual function in BPH and AGA patients using 5α-reductase inhibitors
Irwig MS. Persistent sexual side effects of finasteride: could they be permanent? J Sex Med. 2012;9(11):2927–32. https://doi.org/10.1111/j.1743-6109.2012.02846.x.
Ganzer CA, Jacobs AR, Persistent Sexual IF. Emotional, and cognitive impairment post-finasteride:a survey of men reporting symptoms. Am J Mens Health. 2015;9(3):222–8. https://doi.org/10.1177/1557988314538445.
Kaplan SA, Chung DE, Lee RK, Scofield S, Te AE. A 5-year retrospective analysis of 5alpha-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int J Clin Pract. 2012;66(11):1052–5. https://doi.org/10.1111/j.1742-1241.2012.03010.x.
Mervis JS, Borda LJ, Miteva M. “Post-finasteride syndrome”: what to tell our female patients? Br J Dermatol. 2018; https://doi.org/10.1111/bjd.16658. This meta-analysis investigates the sexual side effects in female patients using 5α-reductase inhibitors off-label for hair loss
Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, et al. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med. 2007;4(6):1708–12. https://doi.org/10.1111/j.1743-6109.2007.00563.x.
Traish AM, Haider KS, Doros G, Haider A. Finasteride, not tamsulosin, increases severity of erectile dysfunction and decreases testosterone levels in men with benign prostatic hyperplasia. Horm Mol Biol Clin Invest. 2015;23(3):85–96. https://doi.org/10.1515/hmbci-2015-0015.
Stojanovic N, Ignjatovic I, Djenic N, Bogdanovic D. Adverse effects of pharmacological therapy of benign prostatic hyperplasia on sexual function in men. Srp Arh Celok Lek. 2015;143(5–6):284–9.
Perez-Mora N, Velasco C, Bermudez F. Oral finasteride presents with sexual-unrelated withdrawal in long-term treated androgenic alopecia in men. Skinmed. 2015;13(3):179–83.
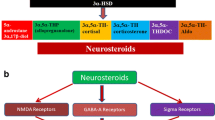
Melcangi RC, Santi D, Spezzano R, Grimoldi M, Tabacchi T, Fusco ML, et al. Neuroactive steroid levels and psychiatric and andrological features in post-finasteride patients. J Steroid Biochem Mol Biol. 2017;171:229–35. https://doi.org/10.1016/j.jsbmb.2017.04.003. This study explores psychiatric and andrological findings in PFS patients and compares their CSF and plasma neurohormonal levels
Walf AA, Kaurejo S, Frye CA. Research brief: self-reports of a constellation of persistent antiandrogenic, estrogenic, physical, and psychological effects of finasteride usage among men. Am J Mens Health. 2018;0(0):1557988317750989. https://doi.org/10.1177/1557988317750989.
Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8(3):872–84. https://doi.org/10.1111/j.1743-6109.2010.02157.x.
Jacobsen SJ, Cheetham TC, Haque R, Shi JM, Loo RK. Association between 5-alpha reductase inhibition and risk of hip fracture. JAMA. 2008;300(14):1660–4. https://doi.org/10.1001/jama.300.14.1660.
Chiriaco G, Cauci S, Mazzon G, Trombetta C. An observational retrospective evaluation of 79 young men with long-term adverse effects after use of finasteride against androgenetic alopecia. Andrology. 2016;4(2):245–50. https://doi.org/10.1111/andr.12147.
Di Loreto C, La Marra F, Mazzon G, Belgrano E, Trombetta C, Cauci S. Immunohistochemical evaluation of androgen receptor and nerve structure density in human prepuce from patients with persistent sexual side effects after finasteride use for androgenetic alopecia. PLoS One. 2014;9(6):e100237. https://doi.org/10.1371/journal.pone.0100237.
Motofei IG, Rowland DL, Georgescu SR, Tampa M, Paunica S, Constantin VD, et al. Post-finasteride adverse effects in male androgenic alopecia: a case report of vitiligo. Skin Pharmacol Physiol. 2017;30(1):42–5. https://doi.org/10.1159/000455972.
Ganzer CA, Jacobs AR. Emotional consequences of finasteride: fool's gold. Am J Mens Health. 2018;12(1):90–5. https://doi.org/10.1177/1557988316631624. This study examines the psychiatric and family history of PFS patients to determine the effect of pre-treatment factors on the course of PFS
Aumuller G, Eicheler W, Renneberg H, Adermann K, Vilja P, Forssmann WG. Immunocytochemical evidence for differential subcellular localization of 5 alpha-reductase isoenzymes in human tissues. Acta Anat. 1996;156(4):241–52.
Cauci S, Chiriaco G, Cecchin E, Toffoli G, Xodo S, Stinco G, et al. Androgen receptor (AR) gene (CAG)n and (GGN)n length polymorphisms and symptoms in young males with long-lasting adverse effects after finasteride use against androgenic alopecia. Sex Med. 2017;5(1):e61–71. https://doi.org/10.1016/j.esxm.2016.11.001.
Motofei IG, Rowland DL, Manea M, Georgescu SR, Paunica I, Sinescu I. Safety profile of finasteride: distribution of adverse effects according to structural and informational dichotomies of the mind/brain. Clin Drug Investig. 2017;37(6):511–7. https://doi.org/10.1007/s40261-017-0501-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jeffrey K Than and Katherine Rodriguez declare no conflicts of interest; Mohit Khera reports research support from the Post-Finasteride Foundation and Boston Scientific, and consulting fees from Boston Scientific, Abbvie, Endo, and Coloplast, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Male Sexual Dysfunction and Disorders
Rights and permissions
About this article
Cite this article
Than, J.K., Rodriguez, K. & Khera, M. Post-finasteride Syndrome: A Review of Current Literature. Curr Sex Health Rep 10, 152–157 (2018). https://doi.org/10.1007/s11930-018-0163-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11930-018-0163-4