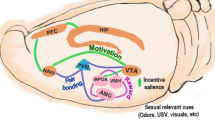
Abstract
Purpose of the Review
In the present manuscript, we review the most important sexual cues in rodents and mammals that influence mate choice. Sexual cues lead to the approach and selection of a partner.
Recent Findings
In both sexes, hormone levels play an important role by increasing the sensitivity towards the sexual signals emitted by the potential partners and determining the expression of sexual signals that allows the potential partner or intra-sexual competitor to identify the reproductive status. Similarly, sexual cues emitted by both sexes can modify the hormonal status of the potential partner or intra-sexual competitors, so that they can be better skilled reproductively for sexual competition.
Summary
Future research should analyze the impact of the use of hormonal contraceptives, since it has been shown that they alter the sexual signals emitted and could influence the selection of partners in humans. In addition, this review will be important for anyone using a rodent model to understand sexual motivation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Darwin C. The descent of man, and selection in selection to sex. London: Murray; 1871.
• Jones AD, Ratterman NL. Mate choice and sexual selection: what have we learned since Darwin? Proc Natl Acad Sci U S A. 2009;106(Suppl 1):10001–8. https://doi.org/10.1073/pnas.0901129106. This study points why does mate choice evolve at all? And second, what factors determine the strength of mate choice.
•• Ågmo A. Functional and dysfunctional sexual behavior a synthesis of neuroscience and comparative psychology. London: Academic Press; 2007. 512 pp. This book reviews the role of sexual incentives and the relationship with the endocrine and central nervous systems.
Agmo A. Sexual motivation. An inquiry into events determining the occurrence of sexual behavior. Behav Brain Res. 1999;105:129–50. https://doi.org/10.1016/S0166-4328(99)00088-1.
Candolin U. The use of multiple cues in mate choice. Biol Rev Camb Philos Soc. 2003;78:575–95. https://doi.org/10.1017/S1464793103006158.
Calhoun JB. The ecology and sociology of the Norway rat. Washington, D.C.: US Government Printing Office; 1962.
Robitaille JA, Bouvet J. Field observations on the social behavior of the Norway rat, Rattus norvegicus (Berkenhout). Biol Behav. 1976;1:289–308.
McClintock MK, Anisko JJ, Adler NT. Group mating among Norway rats. II. The social dynamics of copulation: competition, cooperation, and mate choice. Anim Behav. 1982;30:410–25. https://doi.org/10.1016/S0003-3472(82)80052-3.
Chu X, Ågmo A. Sociosexual behaviors of male rats (Rattus norvegicus) in a seminatural environment. J Comp Psychol. 2015;129:132–44. https://doi.org/10.1037/a0038722.
Chu X, Ågmo A. Sociosexual behaviors during the transition from non-receptivity to receptivity in rats housed in a seminatural environment. Behav Process. 2015;113:24–34. https://doi.org/10.1016/j.beproc.2015.01.001.
Ferreira-Nuño A, Morales-Otal A, Paredes RG, Velázquez-Moctezuma J. Sexual behavior of female rats in a multiple-partner preference test. Horm Behav. 2005;47:290–6. https://doi.org/10.1016/j.yhbeh.2004.11.012.
Snoeren EM, Ågmo A. The role of odors and ultrasonic vocalizations in female rat (Rattus norvegicus) partner choice. J Comp Psychol. 2014;128:367–77. https://doi.org/10.1037/a0036541.
Snoeren EM, Helander LR, Iversen EE, Ågmo A. On the role of individual differences in female odor and ultrasonic vocalizations for male’s choice of partner. Physiol Behav. 2014;132:17–23. https://doi.org/10.1016/j.physbeh.2014.04.048.
Chu AÅ. Sociosexual behaviors in cycling, intact female rats (Rattus norvegicus) housed in a seminatural environment. Behaviour. 2014;151:1143–84. https://doi.org/10.1163/1568539x-00003177.
Chu X, Guarraci FA, Ågmo A. Sociosexual behaviors and reproductive success of rats (Rattus norvegicus) in a seminatural environment. Physiol Behav. 2015;151:46–54. https://doi.org/10.1016/j.physbeh.2015.07.005.
Charlton BD. Experimental tests of mate choice in nonhuman mammals: the need for an integrative approach. J Exp Biol. 2013;216(Pt 7):1127–30. https://doi.org/10.1242/jeb.070318.
Ferreira-Nuño A, Fernández-Soto C, Olayo-Lortia J, Ramirez-Carreto R, Paredes RG, Velázquez-Moctezuma J, et al. Copulatory pattern of male rats in a multiple partner choice arena. J Sex Med. 2010;7:3845–56. https://doi.org/10.1111/j.1743-6109.2010.01746.x.
Ferreira-Nuño A, Cruz-Benites A, Morales-Otal A. Evidence of rejection of a sexually receptive female rat by a group of male rats. Society for Neuroscience. XLVII Annual Meeting. Washington DC, 2017, 11–17.
Morales-Otal A, Olayo-Lortia J, Cruz-Benites A, Ferreira-Nuño A. Exploring the rewarding effect of sexual contacts in the partner preference of the rat. Society for Neuroscience. XLVII Annual Meeting. Washington DC, 2017, 11–17.
Olayo-Lortia J, Cruz-Benites A, Morales-Otal A, Ferreira-Nuño A. Preference for the same receptive female shown by a group of male rats in a multiple partner paradigm. Society for Neuroscience XLVII Annual Meeting. Washington DC, 2017, 11–17.
Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–90. https://doi.org/10.1037/0735-7044.114.5.983.
Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35:85–92. https://doi.org/10.1007/s10519-004-0858-3.
Snoeren EM, Ågmo A. Female ultrasonic vocalizations have no incentive value for male rats. Behav Neurosci. 2013;127:439–50. https://doi.org/10.1037/a0032027.
Thor DH, Flannelly KJ. Peripheral anosmia and social investigatory behavior of the male rat. Behav Biol. 1977;20:128–34. https://doi.org/10.1016/S0091-6773(77)90682-4.
•• Ågmo A, Snoeren EM. A cooperative function for multisensory stimuli in the induction of approach behavior of a potential mate. PLoS One. 2017;12(3):e0174339. https://doi.org/10.1371/journal.pone.0174339. This study highlights the importance of different sexual cues in the initial approach and what makes a conspecific attractive.
Hetta J, Meyerson BJ. Sexual motivation in male rat: methodological study of sex-specific orientation and effects of gonadal hormones. Acta Physiol Scand. 1978;453:5±68.
Hard E, Larsson K. Visual stimulation and mating behavior in male rats. J Comp Physiol Psychol. 1968;66:805±7. https://doi.org/10.1037/h0026557.
•• Kavaliers M, Matta R, Choleris E. Mate-choice copying, social information processing, and the roles of oxytocin. Neurosci Biobehav Rev. 2017;72:232–42. https://doi.org/10.1016/j.neubiorev.2016.12.003. This study analyzes the relationship of mate choice copying with the social learning and social recognition, as well as the neurobiological mechanisms associated with this behavior.
Germain M, Blanchet S, Loyau A, Danchin É. Mate-choice copying in Drosophila melanogaster: impact of demonstration conditions and male-male competition. Behav Process. 2016;125:76–84. https://doi.org/10.1016/j.beproc.2016.02.002.
Dugatkin LA, Godin GJ. Reversal of female mate choice by copying in the guppy: Poecilia reticulata. Proc R Soc Lond B. 1992;249:179–84. https://doi.org/10.1098/rspb.1992.0101.
Dugatkin LA. Sexual selection and imitation: females copy the mate choice of others. Am Nat. 1992;139:1384–9. http://www.jstor.org/stable/2462347
Dugatkin LA, Godin JGJ. Female mate copying in the guppy (Poecilia reticulata): age dependent effects. Behav Ecol. 1993;4:282–92. https://doi.org/10.1093/beheco/4.4.289
Flamarique IN, Bergstrom C, Cheng CL, Reimchen TE. Role of the iridescent eye in stickleback female mate choice. J Exp Biol. 2013;216(Pt15):2806–12. https://doi.org/10.1242/jeb.084889.
Kniel N, Schmitz J, Witte K. Quality of public information matters in mate-choice copying in female zebra finches. Front Zool. 2015;12(26):26. https://doi.org/10.1186/s12983-015-0119-8. eCollection 2015.
Galef BG, Lim TCW, Gilbert GS. Evidence of mate choice copying in Norway rats, Rattus norvegicus. Anim Behav. 2008;75:117–1123. https://doi.org/10.1016/j.anbehav.2007.08.026.
Kavaliers M, Choleris E, Agmo A, Braun WJ, Colwell DD, Muglia LJ, et al. Inadvertent social information and the avoidance of parasitized male mice: a role for oxytocin. Proc Natl Acad Sci U S A. 2006;103:4293–8. https://doi.org/10.1073/pnas.0600410103.
• Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev. 1997;72:283–327. This study analyzes the variation in mating preferences and cost of choosiness among females.
Plath M, Bierbach D. Sex and the public: social eavesdropping, sperm competition risk and male mate choice. Commun Integr Biol. 2011;4:276–80. https://doi.org/10.4161/cib.4.3.14916.
Waynforth D. Mate choice copying in humans. Hum Nat. 2007;18:264–71. https://doi.org/10.1007/s12110-007-9004-2.
Huchard E, Courtiol A, Benavides JA, Knapp LA, Raymond M, Cowlishaw G. Can fertility signals lead to quality signals? Insights from the evolution of primate sexual swellings. Proc Biol Sci. 2009;276:1889–97. https://doi.org/10.1098/rspb.2008.1923.
• Alvergne A, Lummaa V. Does the contraceptive pill alter mate choice in humans? Trends Ecol Evol. 2010;25:171–9. https://doi.org/10.1016/j.tree.2009.08.003. This study shows evidence of how the oral contraceptive pill might significantly alter both female and male mate choices.
Perrett DI, Lee KJ, Penton-Voak I, Rowland D, Yoshikawa S, Burt DM, et al. Effects of sexual dimorphism on facial attractiveness. Nature. 1998;27(394):884–7. https://doi.org/10.1038/29772.
Cobey KD, Buunk AP, Pollet TV, Klipping C, Roberts SC. Men perceive their female partners, and themselves, as more attractive around ovulation. Biol Psychol. 2013;94:513–6. https://doi.org/10.1016/j.biopsycho.2013.09.011.
Fischer J, Semple S, Fickenscher G, Juergens R, Kruse E, Heistermann M, et al. Do women’s voices provide cues of the likelihood of ovulation? The importance of sampling regime. PLoS One. 2011;6:e24490. https://doi.org/10.1371/journal.pone.0024490.
Banai PI. Voice in different phases of menstrual cycle among naturally cycling women and users of hormonal contraceptives. PLoS One. 2017;12:e0183462. https://doi.org/10.1371/journal.pone.0183462.
Puts DA, Bailey DH, Cárdenas RA, Burriss RP, Welling LL, Wheatley JR, et al. Women’s attractiveness changes with estradiol and progesterone across the ovulatory cycle. Horm Behav. 2013;63:13–9. https://doi.org/10.1016/j.yhbeh.2012.11.007.
Cantú SM, Simpson JA, Griskevicius V, Weisberg YJ, Durante KM, Beal DJ. Fertile and selectively flirty: women’s behavior toward men changes across the ovulatory cycle. Psychol Sci. 2014;25:431–8. https://doi.org/10.1177/0956797613508413.
Fink B, Hugill N, Lange BP. Women’s body movements are a potential cue to ovulation. Pers Indiv Differ. 2012;53:759–63. https://doi.org/10.1016/j.paid.2012.06.005.
Miller G, Tybur JM, Jordan BD. Ovulatory cycle effects on tip earnings by lap dancers: economic evidence for human estrus? Evol Hum Behav. 2007;28:375–81. https://doi.org/10.1016/j.evolhumbehav.2007.06.002.
Welling LL, Jones BC, De Bruine LM, Smith FG, Feinberg DR, Little AC, et al. Men report stronger attraction to femininity in women’s faces when their testosterone levels are high. Horm Behav. 2008;54:703–8. https://doi.org/10.1016/j.yhbeh.2008.07.012.
Bobst C, Lobmaier JS. Is preference for ovulatory female’s faces associated with men’s testosterone levels? Horm Behav. 2014;66:487–92. https://doi.org/10.1016/j.yhbeh.2014.06.015.
Bird BM, Welling LL, Ortiz TL, Moreau BJ, Hansen S, Emond M, et al. Effects of exogenous testosterone and mating context on men’s preferences for female facial femininity. Horm Behav. 2016;85:76–85. https://doi.org/10.1016/j.yhbeh.2016.08.003.
O’Connor JJ, Fraccaro PJ, Pisanski K, Tigue CC, Feinberg DR. Men’s preferences for women’s femininity in dynamic cross-modal stimuli. PLoS One. 2013;8:e69531. https://doi.org/10.1371/journal.pone.0069531.
Valentová J, Roberts SC, Havlícek J. Preferences for facial and vocal masculinity in homosexual men: the role of relationship status, sexual restrictiveness, and self-perceived masculinity. Perception. 2013;42(2):187–97.
Roney JR, Simmons ZL. Women’s estradiol predicts preference for facial cues of men’s testosterone. Horm Behav. 2008;53:14–9. https://doi.org/10.1016/j.yhbeh.2007.09.008.
Roney JR, Simmons ZL, Gray PB. Changes in estradiol predict within-women shifts in attraction to facial cues of men’s testosterone. Psychoneuroendocrinol. 2011;36:742–9. https://doi.org/10.1016/j.psyneuen. 2010.10.010.
Welling LL, Jones BC, DeBruine LM, Conway CA, Law Smith MJ, Little AC, et al. Raised salivary testosterone in women is associated with increased attraction to masculine faces. Horm Behav. 2007;52:156–61. https://doi.org/10.1016/j.yhbeh.2007.01.010.
Marcinkowska UM, Ellison PT, Galbarczyk A, Milkowska K, Pawlowski B, Thune I, et al. Lack of support for relation between woman’s masculinity preference, estradiol level and mating context. Horm Behav. 2016;78:1–7. https://doi.org/10.1016/j.yhbeh.2015.10.012.
Cobey KD, Havlíček J, Klapilová K, Roberts SC. Hormonal contraceptive use during relationship formation and sexual desire during pregnancy. Arch Sex Behav. 2016;45:2117–22. https://doi.org/10.1007/s10508-015-0662-6.
Bobst C, Sauter S, Foppa A, Lobmaier JS. Early follicular testosterone level predicts preference for masculinity in male faces—but not for women taking hormonal contraception. Psychoneuroendocrinol. 2014;41:142–50.
Cobey KD, Klipping C, Buunk AP. Hormonal contraceptive use lowers female intrasexual competition in pair-bonded women. Evol Hum Behav. 2013;2013(34):294–8. https://doi.org/10.1016/j.evolhumbehav.2013.04.003.
Haselton MG, Mortezaie M, Pillsworth EG, Bleske-Rechek A, Frederick DA. Ovulatory shifts in human female ornamentation: near ovulation, women dress to impress. Horm Behav. 2007;51:40–5.
Saad G, Stenstrom E. Calories, beauty, and ovulation: the effects of the menstrual cycle on food and appearance-related consumption. J Consum Psychol. 2012;22:102–13. https://doi.org/10.1016/j.jcps.2011.10.001.
Blake KR, Bastian B, O’Dean SM, Denson TF. High estradiol and low progesterone are associated with high assertiveness in women. Psychoneuroendocrinol. 2017;75:91–9. https://doi.org/10.1016/j.psyneuen.2016.10.008.
Beall AT, Tracy JL. Women are more likely to wear red or pink at peak fertility. Psychol Sci. 2013;24:1837–41. https://doi.org/10.1177/0956797613476045.
Pazda AD, Prokop P, Elliot AJ. Red and romantic rivalry: viewing another woman in red increases perceptions of sexual receptivity, derogation, and intentions to mate-guard. Personal Soc Psychol Bull. 2014;40:1260–9. https://doi.org/10.1177/0146167214539709.
Tracy JL, Beall AT. The impact of weather on women’s tendency to wear red or pink when at high risk for conception. PLoS One. 2014;9(2):e88852. https://doi.org/10.1371/journal.pone.
Elliot AJ, Pazda AD. Dressed for sex: red as a female sexual signal in humans. PLoS One. 2012;7:e34607. https://doi.org/10.1371/journal.pone.0034607.
Prokop P, Hromada M. Women use red in order to attract mates. Ethol Sociobiol. 2013;119:605–13. https://doi.org/10.1111/eth.12102.
Prokop P, Pazda AD. Women’s red clothing can increase mate-guarding from their male partner. Personal Individ Differ. 2016;98:114–7. https://doi.org/10.1016/j.paid.2016.04.021.
Guéguen N, Jacob C. Clothing color and tipping: gentlemen patrons give more tips to waitresses with red clothes. Int J Hospital Manag. 2012;31:1333–5. https://doi.org/10.1177/1096348012442546.
Elliot AJ, Kayser DN, Greitemeyer T, Lichtenfeld S, Gramzow RH, Maier MA, et al. Red, rank, and romance in women viewing men. J Exp Psychol Gen. 2010;139:399–417. https://doi.org/10.1037/a0019689.
Fink B, Bunse L, Matts PJ, D’Emiliano D. Visible skin colouration predicts perception of male facial age, health and attractiveness. Int J Cosmet Sci. 2012;34:307–10. https://doi.org/10.1111/j.1468-2494.2012.00724.x.
Gildersleeve KA, Haselton MG, Larson CM, Pillsworth EG. Body odor attractiveness as a cue of impending ovulation in women: evidence from a study using hormone-confirmed ovulation. Horm Behav. 2012;61:157–66. https://doi.org/10.1016/j.yhbeh.2011.11.005.
Maner JK, McNulty JK. Attunement to the fertility status of same-sex rivals: women’s testosterone responses to olfactory ovulation cues. Evol Hum Behav. 2013;34:412–8. https://doi.org/10.1016/j.evolhumbehav.2013.07.005.
Jones BC, De Bruine LM, Little AC, Burriss RP, Feinberg DR. Social transmission of face preferences among humans. Proc Biol Sci. 2007;274:899–903. https://doi.org/10.1098/rspb.2006.0205.
Hill SE, Buss DM. The mere presence of opposite-sex others on judgments of sexual and romantic desirability: opposite effects for men and women. Personal Soc Psychol Bull. 2008;34:635–47. https://doi.org/10.1177/0146167207313728.
Funding
The research was supported by CONACYT grant 253631, Fronteras 374 and PAPIIT 203518.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Preclinical and Psychophysiology
Rights and permissions
About this article
Cite this article
Ferreira-Nuño, A., Olayo-Lortia, J., Cruz-Benites, A. et al. Sexual Incentive and Choice. Curr Sex Health Rep 10, 132–141 (2018). https://doi.org/10.1007/s11930-018-0158-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11930-018-0158-1