Abstract
Purpose of Review
The following review discusses the therapeutic potential of targeting the autonomic nervous system (ANS) for osteoarthritis (OA) treatment and encourages the field to consider the candidacy of bioelectronic medicine as a novel OA treatment strategy.
Recent Findings
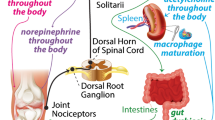
The study of OA pathogenesis has focused on changes occurring at the joint level. As such, treatments for OA have been aimed at the local joint environment, intending to resolve local inflammation and decrease pain. However, OA pathogenesis has shown to be more than joint wear and tear. Specifically, OA-related peripheral and central sensitization can prompt neuroplastic changes in the nervous system beyond the articular joint. These neuroplastic changes may alter physiologic systems, like the neuroimmune axis. In this way, OA and related comorbidities may share roots in the form of altered neuroimmune communication and autonomic dysfunction.
Summary
ANS modulation may be able to modify OA pathogenesis or reduce the impact of OA comorbidities. Moreover, blocking chronic nociceptive drive from the joint may help to prevent maladaptive nervous system plasticity in OA.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–23.
Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochimica et Biophysica Acta (BBA) - molecular basis of disease [Internet]. 2016;1862:576–91. Available from: https://www.sciencedirect.com/science/article/pii/S0925443916000041. Accessed 27 Jul 2022
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–92.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707.
Chakrabarti S, Jadon DR, Bulmer DC, Smith ESJ. Human osteoarthritic synovial fluid increases excitability of mouse dorsal root ganglion sensory neurons: an in-vitro translational model to study arthritic pain. Rheumatology (United Kingdom). 2020. https://doi.org/10.1093/rheumatology/kez331
Kwok CHT, Kohro Y, Mousseau M, O’Brien MS, Matyas JR, McDougall JJ, et al. Role of primary afferents in arthritis induced spinal microglial reactivity. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.626884
Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain U S. 1999;79:75–82.
Mease PJ, Hanna S, Frakes EP, Altman RD. Pain mechanisms in osteoarthritis: understanding the role of central pain and current approaches to its treatment. J Rheumatol Canada. 2011;38:1546–51.
Ivanavicius SP, Ball AD, Heapy CG, Westwood RF, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain U S. 2007;128:272–82.
Ohtori S, Orita S, Yamashita M, Ishikawa T, Ito T, Shigemura T, et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med J. 2012;53:801–5.
McDougall JJ. Osteoarthritis is a neurological disease – an hypothesis. Osteoarthr Cartil Open [Internet]. 2019;1:100005. Available from: https://www.sciencedirect.com/science/article/pii/S2665913119300056. Accessed 06 Jul 2022
Yeater TD, Cruz CJ, Cruz-Almeida Y, Allen KD. Autonomic nervous system dysregulation and osteoarthritis pain: mechanisms, measurement, and future outlook. Curr Rheumatol Rep United States. 2022. https://doi.org/10.1007/s11926-022-01071-9
• Berenbaum F, Meng QJ. The brain-joint axis in osteoarthritis: nerves, circadian clocks and beyond. Nat Rev Rheumatol. 2016. This review proposes the brain-joint axis as a physiological axis that becomes dysregulated in osteoarthritis and contributes to the systemic consequences of OA pathogenesis. https://doi.org/10.1038/nrrheum.2016.93
• Courties A, Sellam J, Berenbaum F. Role of the autonomic nervous system in osteoarthritis. Best Pract Res Clin Rheumatol. 2017. This review discusses potential crosstalk mechanisms between the autonomic nervous system and osteoarthritis pathogenesis. https://doi.org/10.1016/j.berh.2018.04.001
Yeater TD, Zubcevic J, Allen KD. Measures of cardiovascular function suggest autonomic nervous system dysregulation after surgical induction of joint injury in the male Lewis rat. Osteoarthr Cartil England. 2022;30:586–95.
Adlan AM, Veldhuijzen van Zanten JJCS, Lip GYH, Paton JFR, Kitas GD, Fisher JP. Cardiovascular autonomic regulation, inflammation and pain in rheumatoid arthritis. Auton Neurosci. 2017;208:137–45.
Alen NV, Deer LK, Hostinar CE. Autonomic nervous system activity predicts increasing serum cytokines in children. Psychoneuroendocrinology [Internet]. 2020;119:104745. Available from: https://www.sciencedirect.com/science/article/pii/S0306453020301645. Accessed 08 Jun 2022
Ghia J-E, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–18.
Abuín-Porras V, Clemente-Suárez VJ, Jaén-Crespo G, Navarro-Flores E, Pareja-Galeano H, Romero-Morales C. Effect of physiotherapy treatment in the autonomic activation and pain perception in male patients with non-specific subacute low back pain. J Clin Med. 2021;10:8. https://doi.org/10.3390/jcm10081793
Fu Q, Levine BD. Exercise and the autonomic nervous system. Handb Clin Neurol Netherlands. 2013;117:147–60.
Koopman FA, van Maanen MA, Vervoordeldonk MJ, Tak PP. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J Intern Med England. 2017;282:64–75.
Zeng C, Li H, Yang T, Deng Z, Yang Y, Zhang Y, et al. Electrical stimulation for pain relief in knee osteoarthritis: systematic review and network meta-analysis. Osteoarthr Cartil England. 2015;23:189–202.
Lee R, Kean WF. Obesity and knee osteoarthritis. Inflammopharmacology. Switzerland; 2012;20:53–8.
Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev. 2014;15:578–86.
Rossi RC, Vanderlei LCM, Gonçalves ACCR, Vanderlei FM, Bernardo AFB, Yamada KMH, et al. Impact of obesity on autonomic modulation, heart rate and blood pressure in obese young people. Auton Neurosci Netherlands. 2015;193:138–41.
Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114:1804–14.
Zhang Y-M, Wang J, Liu X-G. Association between hypertension and risk of knee osteoarthritis: a meta-analysis of observational studies. Medicine [Internet]. Wolters Kluwer Health. 2017;96:e7584–e7584. Available from: https://pubmed.ncbi.nlm.nih.gov/28796041. Accessed 02 Oct 2021
Baker SE, Limberg JK, Dillon GA, Curry TB, Joyner MJ, Nicholson WT. Aging alters the relative contributions of the sympathetic and parasympathetic nervous system to blood pressure control in women. Hypertension. 2018;72:1236–42.
Veronese N, Cooper C, Reginster J-Y, Hochberg M, Branco J, Bruyère O, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. 2019;49:9–19.
Carnethon MR, Jacobs DR, Sidney S, Liu K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes. Diabetes Care [Internet]. 2003;26:3035 LP – 3041. Available from: http://care.diabetesjournals.org/content/26/11/3035.abstract. Accessed 08 Oct 2021
Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol [Internet]. 2016;12:412–20. Available from: https://doi.org/10.1038/nrrheum.2016.65
Parashar R, Amir M, Pakhare A, Rathi P, Chaudhary L. Age related changes in autonomic functions. J Clin Diagn Res. 2016;10:CC11-5.
Wallace IJ, Bendele AM, Riew G, Frank EH, Hung H-H, Holowka NB, et al. Physical inactivity and knee osteoarthritis in guinea pigs. Osteoarthr Cartil England. 2019;27:1721–8.
Exercise is essential for osteoarthritis: the many benefits of physical activity. Journal of Orthopaedic \& Sports Physical Therapy [Internet]. 2018;48:448. Available from: https://doi.org/10.2519/jospt.2018.0507
Hughson RL, Shoemaker JK. Autonomic responses to exercise: deconditioning/inactivity. Autonomic Neuroscience [Internet]. 2015;188:32–5. Available from: https://www.sciencedirect.com/science/article/pii/S1566070214001672. Accessed 02 Oct 2021
Goldsmith RL, Bloomfield DM, Rosenwinkel ET. Exercise and autonomic function. Coron Artery Dis England. 2000;11:129–35.
Urban H, Little CB. The role of fat and inflammation in the pathogenesis and management of osteoarthritis. Rheumatology [Internet]. 2018;57:iv10–21. Available from: https://doi.org/10.1093/rheumatology/kex399
Maser RE, Lenhard MJ. An overview of the effect of weight loss on cardiovascular autonomic function. Curr Diabetes Rev United Arab Emirates. 2007;3:204–11.
Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol United States. 2001;18:415–8.
Noller CM, Levine YA, Urakov TM, Aronson JP, Nash MS. Vagus nerve stimulation in rodent models: an overview of technical considerations. Front Neurosci. 2019;13. https://doi.org/10.3389/fnins.2019.00911
Goldring MB. Articular cartilage degradation in osteoarthritis. HSS J. 2012;8:7–9.
Li Y-S, Luo W, Zhu S-A, Lei G-H. T Cells in osteoarthritis: alterations and beyond. Front Immunol. 2017;8:356.
Zhang H, Lin C, Zeng C, Wang Z, Wang H, Lu J, et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis [Internet]. 2018;77:1524 LP – 1534. Available from: http://ard.bmj.com/content/77/10/1524.abstract. Accessed 08 Oct 2021
Saito I, Koshino T, Nakashima K, Uesugi M, Saito T. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthr Cartil England. 2002;10:156–62.
Rotenberg S, McGrath JJ. Inter-relation between autonomic and HPA axis activity in children and adolescents. Biol Psychol [Internet]. 2016/02/02. 2016;117:16–25. Available from: https://pubmed.ncbi.nlm.nih.gov/26835595. Accessed 24 May 2022
Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603–21.
Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018. https://doi.org/10.3389/fnins.2018.00049
Tracey KJ. The inflammatory reflex. Nature. 2002. https://doi.org/10.1038/nature01321
Teh YC, Ding JL, Ng LG, Chong SZ. Capturing the fantastic voyage of monocytes through time and space. Front Immunol [Internet]. 2019;10. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.00834. Accessed 27 Jun 2022
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014. https://doi.org/10.1155/2014/561459
Pickering AE, Boscan P, Paton JFR. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol. 2003. https://doi.org/10.1113/jphysiol.2003.046615
Yeater TD, Clark DJ, Hoyos L, Valdes-Hernandez PA, Peraza JA, Allen KD, et al. Chronic pain is associated with reduced sympathetic nervous system reactivity during simple and complex walking tasks: potential cerebral mechanisms. Chronic Stress [Internet]. SAGE Publications Inc. 2021;5:24705470211030270. Available from: https://doi.org/10.1177/24705470211030273. Accessed 25 Apr 2022
Hohenschurz-Schmidt DJ, Calcagnini G, Dipasquale O, Jackson JB, Medina S, O’Daly O, et al. Linking pain sensation to the autonomic nervous system: the role of the anterior cingulate and periaqueductal gray resting-state networks. Front Neurosci [Internet]. 2020;14. Available from: https://www.frontiersin.org/article/10.3389/fnins.2020.00147. Accessed 02 Jun 2022
Caravaca AS, Gallina AL, Tarnawski L, Tracey KJ, Pavlov VA, Levine YA, et al. An effective method for acute vagus nerve stimulation in experimental inflammation. Front Neurosci. 2019;13:877. https://doi.org/10.3389/fnins.2019.00877
Valdes-Ferrer S, Rosas-Ballina M, Olofsson P, Chavan S, Tracey K. Vagus nerve stimulation produces an anti-inflammatory monocyte phenotype in blood. (138.25). J Immunol. 2010;184(1 Supplement):138.25 LP-138.25. https://doi.org/10.4049/jimmunol.184.Supp.138.25
Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. In: Autonomic Neuroscience: Basic and Clinical. 2000. https://doi.org/10.1016/S1566-0702(00)00233-2
Mion F, Pellissier S, Garros A, Damon H, Roman S, Bonaz B. Transcutaneous auricular vagus nerve stimulation for the treatment of irritable bowel syndrome: a pilot, open-label study. Bioelectron Med (Lond). 2020. https://doi.org/10.2217/bem-2020-0004
Marsal S, Corominas H, Lopez Lasanta M, Reina-Sanz D, Perez-Garcia C, Borrell Paños H, et al. Sat0133 pilot clinical study of a non-invasive auricular vagus nerve stimulation device in patients with rheumatoid arthritis. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-eular.3315
•• Courties A, Deprouw C, Maheu E, Gibert E, Gottenberg J-E, Champey J, et al. Effect of transcutaneous vagus nerve stimulation in erosive hand osteoarthritis: results from a pilot trial. J Clin Med. 2022;11:4. This study is the first to demonstrate vagus nerve stimulation as an effective treatment strategy for osteoarthritis. https://doi.org/10.3390/jcm11041087
Komegae EN, Farmer DGS, Brooks VL, McKinley MJ, McAllen RM, Martelli D. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun. 2018. https://doi.org/10.1016/j.bbi.2018.06.005
Grunke M, Schulze-Koops H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann Rheum Dis [Internet]. BMJ Group; 2006;65:555–6. Available from: https://pubmed.ncbi.nlm.nih.gov/16531556. Accessed 01 Feb 2021
Chisari E, Yaghmour KM, Khan WS. The effects of TNF-alpha inhibition on cartilage: a systematic review of preclinical studies. Osteoarthritis Cartilage. 2020. https://doi.org/10.1016/j.joca.2019.09.008
Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010. https://doi.org/10.1016/j.joca.2010.08.016
Huston JM, Rosas-Ballina M, Xue X, Dowling O, Ochani K, Ochani M, et al. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol. 2009. https://doi.org/10.4049/jimmunol.0802684
Zhao X, Gu M, Xu X, Wen X, Yang G, Li L, et al. CCL3/CCR1 mediates CD14+CD16− circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthritis Cartilage [Internet]. 2020;28:613–25. Available from: https://www.sciencedirect.com/science/article/pii/S1063458420300352. Accessed 28 Jun 2022
Thomson A, Hilkens CMU. Synovial macrophages in osteoarthritis: the key to understanding pathogenesis? Front Immunol. 2021;12:678757.
Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, Zitnik R, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One. 2014. https://doi.org/10.1084/jem.20040463
Cremers NAJ, van den Bosch MHJ, van Dalen S, di Ceglie I, Ascone G, van de Loo F, et al. S100A8/A9 increases the mobilization of pro-inflammatory Ly6Chigh monocytes to the synovium during experimental osteoarthritis. Arthritis Res Ther [Internet]. 2017;19:217. Available from: https://doi.org/10.1186/s13075-017-1426-6
Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005. https://doi.org/10.1084/jem.20040463
Molnar V, Matišić V, Kodvanj I, Bjelica R, Jeleč Ž, Hudetz D, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021;22:17. https://doi.org/10.3390/ijms22179208
• Bassi GS, Ulloa L, Santos VR, del Vecchio F, Delfino-Pereira P, Rodrigues GJ, et al. Cortical stimulation in conscious rats controls joint inflammation. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:201–13. This study demonstrates how vagus nerve stimulation can activate the sympathetic nervous system, specifically cortical sympatho-excitatory brain regions, to lower joint inflammation in rats
• Bassi GS, Dias DPM, Franchin M, Talbot J, Reis DG, Menezes GB, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017;64:330–43. This study demonstrates how vagus nerve stimulation can activate the sympathetic control centers in the brain to lower joint inflammation in rats
Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci Lett Ireland. 2005;379:174–9.
Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology England. 2008;33:1884–95.
Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther United States. 2006;318:890–8.
Eitner A, Pester J, Nietzsche S, Hofmann GO, Schaible H-G. The innervation of synovium of human osteoarthritic joints in comparison with normal rat and sheep synovium. Osteoarthritis Cartilage [Internet]. 2013;21:1383–91. Available from: https://www.sciencedirect.com/science/article/pii/S1063458413008546. Accessed 09 Jun 2022
Kaps J, Straub RH, Böhm M, Beckmann J, Grifka J, Grässel S. The role of norepinephrine in human articular chondrocytes. Osteoarthritis Cartilage [Internet]. 2012;20:S144–5. Available from: http://www.sciencedirect.com/science/article/pii/S1063458412002774. Accessed 01 Feb 2021
Lorenz J, Schäfer N, Bauer R, Jenei-Lanzl Z, Springorum RH, Grässel S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthr Cartil. 2016. https://doi.org/10.1016/j.joca.2015.08.007
Hwang HS, Lee MH, Go DJ, Kim HA. Norepinephrine modulates IL-1β-induced catabolic response of human chondrocytes. BMC Musculoskelet Disord. 2021;22:724.
Evrengül H, Dursunoglu D, Cobankara V, Polat B, Seleci D, Kabukçu S, et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol Int. 2004.
Provan SA, Olstad DS, Solberg EE, Smedslund G, Dagfinrud H. Evidence of reduced parasympathetic autonomic regulation in inflammatory joint disease: a meta-analyses study. Semin Arthritis Rheum. 2018. https://doi.org/10.1016/j.semarthrit.2017.11.010
Rösch G, el Bagdadi K, Muschter D, Taheri S, Dorn C, Meurer A, et al. Sympathectomy aggravates subchondral bone changes during osteoarthritis progression in mice without affecting cartilage degeneration or synovial inflammation. Osteoarthr Cartil [Internet]. 2022;30:461–74. Available from: https://www.sciencedirect.com/science/article/pii/S1063458421009791. Accessed 27 Jul 2022
Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci [Internet]. 2018;12. Available from: https://www.frontiersin.org/article/10.3389/fnbeh.2018.00127. Accessed 24 May 2022
Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol [Internet]. 2002;20:125–63. Available from: https://doi.org/10.1146/annurev.immunol.20.082401.104914. Accessed 04 Oct 2021
Sen Y, Aygun D, Yilmaz E, Ayar A. Children and adolescents with obesity and the metabolic syndrome have high circulating cortisol levels. Neuro Endocrinol Lett Sweden. 2008;29:141–5.
Nishimura H, Kawasaki M, Matsuura T, Suzuki H, Motojima Y, Baba K, et al. Acute mono-arthritis activates the neurohypophysial system and hypothalamo-pituitary adrenal axis in rats. Front Endocrinol (Lausanne). 2020;11:43.
Staniszewski K, Lygre H, Bifulco E, Kvinnsland S, Willassen L, Helgeland E, et al. Temporomandibular disorders related to stress and HPA-axis regulation. Pain Res Manag. 2018;2018:7020751.
Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Penninx BWJH, et al. Reduced hypothalamic-pituitary-adrenal axis activity in chronic multi-site musculoskeletal pain: partly masked by depressive and anxiety disorders. BMC Musculoskelet Disord. 2014;15:227.
Kim H-G, Cheon E-J, Bai D-S, Lee YH, Koo B-H. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 2018;15:235–45.
Calogero AE, Sternberg EM, Bagdy G, Smith C, Bernardini R, Aksentijevich S, et al. Neurotransmitter-lnduced hypothalamic-pituitary-adrenal axis responsiveness is defective in inflammatory disease-susceptible lewis rats: in vivo and in vitro studies suggesting globally defective hypothalamic secretion of corticotropin-releasing hormone. Neuroendocrinology. 1992. https://doi.org/10.1159/000126173
Morand EF, Leech M. Hypothalamic-pituitary-adrenal axis regulation of inflammation in rheumatoid arthritis. Immunol Cell Biol. 2001. https://doi.org/10.1046/j.1440-1711.2001.01028.x
Paschali M, Lazaridou A, Paschalis T, Moradian JR, Sadora J, Vilsmark ES, et al. Individual variation in diurnal cortisol in patients with knee osteoarthritis: clinical correlates. Int J Psychophysiol. 2021;167:1–6.
Mickle AM, Garvan C, Service C, Pop R, Marks J, Wu S, et al. Relationships between pain, life stress, sociodemographics, and cortisol: contributions of pain intensity and financial satisfaction. Chronic Stress (Thousand Oaks). 2020;4:2470547020975758.
Villafañe JH, Pedersini P, Bertozzi L, Drago L, Fernandez-Carnero J, Bishop MD, et al. Exploring the relationship between chronic pain and cortisol levels in subjects with osteoarthritis: results from a systematic review of the literature. Osteoarthr Cartil England. 2020;28:572–80.
de Herdt V, Puimege L, de Waele J, Raedt R, Wyckhuys T, el Tahry R, et al. Increased rat serum corticosterone suggests immunomodulation by stimulation of the vagal nerve. J Neuroimmunol. 2009.
Hosoi T, Okuma Y, Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1β expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol. 2000.
Collins KH, Paul HA, Reimer RA, Seerattan RA, Hart DA, Herzog W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthr Cartil. 2015.
Metcalfe D, Harte AL, Aletrari MO, al Daghri NM, al Disi D, Tripathi G, et al. Does endotoxaemia contribute to osteoarthritis in obese patients? Clin Sci. 2012.
Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SCJ, et al. Unhealthy lifestyle and gut dysbiosis: a better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front Endocrinol (Lausanne). 2021;12:667066.
Zhou H, Liang H, Li ZF, Xiang H, Liu W, Li JG. Vagus nerve stimulation attenuates intestinal epithelial tight junctions disruption in endotoxemic mice through α7 nicotinic acetylcholine receptors. Shock. 2013.
Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci [Internet]. 2013;9:99–107. Available from: https://pubmed.ncbi.nlm.nih.gov/24353483. Accessed 20 Jul 2021
Bonaz B. Parameters matter: modulating cytokines using nerve stimulation. Bioelectron Med. 2020;6:12.
Monaco A, Cattaneo R, Mesin L, Ciarrocchi I, Sgolastra F, Pietropaoli D. Dysregulation of the autonomous nervous system in patients with temporomandibular disorder: a pupillometric study. PLoS One. 2012;7:e45424.
Martins DF, Viseux FJF, Salm DC, Ribeiro ACA, da Silva HKL, Seim LA, et al. The role of the vagus nerve in fibromyalgia syndrome. Neurosci Biobehav Rev United States. 2021;131:1136–49.
Bagnato GL, Miceli G, Marino N, Sciortino D, Bagnato GF. Pulsed electromagnetic fields in knee osteoarthritis: a double blind, placebo-controlled, randomized clinical trial. Rheumatology (Oxford). 2016;55:755–62.
Joseph L, Butera RJ. Unmyelinated Aplysia nerves exhibit a nonmonotonic blocking response to high-frequency stimulation. IEEE Trans Neural Syst Rehabil Eng United States. 2009;17:537–44.
Franke M, Bhadra N, Bhadra N, Kilgore K. Direct current contamination of kilohertz frequency alternating current waveforms. J Neurosci Methods. 2014;232:74–83.
Ackermann DMJ, Ethier C, Foldes EL, Oby ER, Tyler D, Bauman M, et al. Electrical conduction block in large nerves: high-frequency current delivery in the nonhuman primate. Muscle Nerve. 2011;43:897–9.
Avendano-Coy J, Serrano-Munoz D, Taylor J, Goicoechea-Garcia C, Gomez-Soriano J. Peripheral nerve conduction block by high-frequency alternating currents: a systematic review. IEEE Trans Neural Syst Rehabil Eng United States. 2018;26:1131–40.
Soin A, Shah NS, Fang Z-P. High-frequency electrical nerve block for postamputation pain: a pilot study. Neuromodulation United States. 2015;18:196–7.
Dewberry LS, Dru AB, Gravenstine M, Nguyen B, Anderson J, Vaziri S, et al. Partial high frequency nerve block decreases neuropathic signaling following chronic sciatic nerve constriction injury. J Neural Eng. England. 2021;18.
Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. United States; 2015;18:24–32; discussion 32.
Martin SC, Macey AR, Raghu A, Edwards T, Watson C, Bojanić S, et al. Dorsal root ganglion stimulation for the treatment of chronic neuropathic knee pain. World Neurosurg [Internet]. 2020;143:e303–8. Available from: https://www.sciencedirect.com/science/article/pii/S1878875020316235. Accessed 28 Jul 2022
Pan B, Zhang Z, Chao D, Hogan QH. Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation: Technology at the Neural Interface [Internet]. 2018;21:247–53. Available from: https://www.sciencedirect.com/science/article/pii/S1094715921022029. Accessed 28 Jul 2022
Yu G, Segel I, Zhang Z, Hogan QH, Pan B. Dorsal root ganglion stimulation alleviates pain-related behaviors in rats with nerve injury and osteoarthritis. Anesthesiology. 2020;133:408–25.
Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am. 2008;34:623–43.
McDougall JJ, Karimian SM, Ferrell WR. Prolonged alteration of vasoconstrictor and vasodilator responses in rat knee joints by adjuvant monoarthritis. Exp Physiol England. 1995;80:349–57.
Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018;18:1048–67.
Guan Y, Wacnik PW, Yang F, Carteret AF, Chung C-Y, Meyer RA, et al. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology United States. 2010;113:1392–405.
Li S, Farber JP, Linderoth B, Chen J, Foreman RD. Spinal cord stimulation with ‘conventional clinical’ and higher frequencies on activity and responses of spinal neurons to noxious stimuli: an animal study. Neuromodulation United States. 2018;21:440–7.
Crosby ND, Goodman Keiser MD, Smith JR, Zeeman ME, Winkelstein BA. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation. United States; 2015;18:1–8; discussion 8.
Wallin J, Fiskå A, Tjølsen A, Linderoth B, Hole K. Spinal cord stimulation inhibits long-term potentiation of spinal wide dynamic range neurons. Brain Res Netherlands. 2003;973:39–43.
Funding
Authors for this publication are supported by the national institute of arthritis and musculoskeletal and skin diseases of the national institutes of health under award number R01AR071431, R01AR071431-S1, the national science foundation under fellowship grant number DGE-1842473, and a graduate student fellowship award from the University of Florida.
Author information
Authors and Affiliations
Contributions
CJC and KDA conceptualized this review. CJC drafted the manuscript, with critical contributions from LSD, KJO, and KDA. All authors approved the final version of the manuscript and figures.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not include any human or animal research that was performed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoarthritis
Key Points
• Dysfunction of the autonomic nervous system may be a key driver of OA comorbidities and thus may be a critical component of OA’s effects on overall health.
• Vagus nerve stimulation could activate the neuroimmune axis to decrease OA pathogenesis or reduce the risk of OA pathogenesis.
• Blocking chronic OA pain could protect against pathologic shifts in the brain-joint axis and decrease the risk of chronic comorbid disease.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cruz, C.J., Dewberry, L.S., Otto, K.J. et al. Neuromodulation as a Potential Disease-Modifying Therapy for Osteoarthritis. Curr Rheumatol Rep 25, 1–11 (2023). https://doi.org/10.1007/s11926-022-01094-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-022-01094-2