Abstract
Purpose of the Review
Systemic lupus erythematosus (SLE) is driven by nucleic acid-containing antigens that stimulate endosomal TLRs. We review new advances in our understanding of how TLR7 signaling in B cells drives autoimmunity.
Recent Findings
Pathogenic B cell responses to TLR7 engagement are shaped by the disease-associated cytokine environment. TLR7, IFNγ, and IL-21 together promote the formation of autoreactive germinal centers and the ABC/DN2 B cell subset. BAFF and type 1 IFNs enhance autoantibody production from transitional B cells in concert with TLR7. TLR7 signaling components STAT1, BANK1, IRF5, SLC15A4, and CXorf21/TASL are associated genetically with SLE and important for lupus development in mice, while role of T-bet is controversial. Proper control of TLR7 trafficking by UNC93B1, syntenin-1, and αvβ3 integrin is critical for preventing autoimmunity.
Summary
A better understanding of TLR7 signaling has revealed potential new therapeutic approaches for SLE, several of which are being tested in animal models or clinical trials.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18(6):871–82. https://doi.org/10.1038/nm.2752.
Atisha-Fregoso Y, Toz B, Diamond B. Meant to B: B cells as a therapeutic target in systemic lupus erythematosus. J Clin Invest. 2021;131(12). https://doi.org/10.1172/JCI149095.
Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–7. https://doi.org/10.1038/416603a.
Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202(9):1171–7. https://doi.org/10.1084/jem.20050630.
Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103(26):9970–5. https://doi.org/10.1073/pnas.0603912103.
Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312(5780):1669–72. https://doi.org/10.1126/science.1124978.
Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27(5):801–10. https://doi.org/10.1016/j.immuni.2007.09.009.
Yokogawa M, Takaishi M, Nakajima K, Kamijima R, Fujimoto C, Kataoka S, et al. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: a new model of systemic Lupus erythematosus. Arthritis Rheumatol. 2014;66(3):694–706. https://doi.org/10.1002/art.38298.
Chodisetti SB, Fike AJ, Domeier PP, Singh H, Choi NM, Corradetti C, et al. Type II but Not Type I IFN Signaling Is Indispensable for TLR7-Promoted Development of Autoreactive B Cells and Systemic Autoimmunity. J Immunol. 2020;204(4):796–809. https://doi.org/10.4049/jimmunol.1901175.
Hwang SH, Lee H, Yamamoto M, Jones LA, Dayalan J, Hopkins R, et al. B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. J Immunol. 2012;189(12):5786–96. https://doi.org/10.4049/jimmunol.1202195.
Jackson SW, Scharping NE, Kolhatkar NS, Khim S, Schwartz MA, Li QZ, et al. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol. 2014;192(10):4525–32. https://doi.org/10.4049/jimmunol.1400098.
Soni C, Wong EB, Domeier PP, Khan TN, Satoh T, Akira S, et al. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J Immunol. 2014;193(9):4400–14. https://doi.org/10.4049/jimmunol.1401720.
• Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19). https://doi.org/10.1126/sciimmunol.aap8855. This study demonstrates that human immune cells can express TLR7 from both X chromosomes, and those cells that do are more responsive to TLR7 engagement. This suggests that increased TLR7 expression in females may contribute to the strong female predominance in SLE.
Yu B, Qi Y, Li R, Shi Q, Satpathy AT, Chang HY. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell. 2021;184(7):1790–803.e17. https://doi.org/10.1016/j.cell.2021.02.015.
Nundel K, Green NM, Shaffer AL, Moody KL, Busto P, Eilat D, et al. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J Immunol. 2015;194(6):2504–12. https://doi.org/10.4049/jimmunol.1402425.
Tilstra JS, John S, Gordon RA, Leibler C, Kashgarian M, Bastacky S, et al. B cell-intrinsic TLR9 expression is protective in murine lupus. J Clin Invest. 2020;130(6):3172–87. https://doi.org/10.1172/JCI132328.
Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-gamma and systemic autoimmunity. Discov Med. 2013;16(87):123–31.
Domeier PP, Chodisetti SB, Soni C, Schell SL, Elias MJ, Wong EB, et al. IFN-gamma receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med. 2016;213(5):715–32. https://doi.org/10.1084/jem.20151722.
Jackson SW, Jacobs HM, Arkatkar T, Dam EM, Scharping NE, Kolhatkar NS, et al. B cell IFN-gamma receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med. 2016;213(5):733–50. https://doi.org/10.1084/jem.20151724.
Chodisetti SB, Fike AJ, Domeier PP, Choi NM, Soni C, Rahman ZSM. TLR7 Negatively Regulates B10 Cells Predominantly in an IFNgamma Signaling Dependent Manner. Front Immunol. 2020;11:1632. https://doi.org/10.3389/fimmu.2020.01632.
Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunol Res. 2013;55(1–3):210–6. https://doi.org/10.1007/s12026-012-8365-8.
Du SW, Arkatkar T, Jacobs HM, Rawlings DJ, Jackson SW. Generation of functional murine CD11c(+) age-associated B cells in the absence of B cell T-bet expression. Eur J Immunol. 2019;49(1):170–8. https://doi.org/10.1002/eji.201847641.
Ricker E, Manni M, Flores-Castro D, Jenkins D, Gupta S, Rivera-Correa J, et al. Altered function and differentiation of age-associated B cells contribute to the female bias in lupus mice. Nat Commun. 2021;12(1):4813. https://doi.org/10.1038/s41467-021-25102-8 . This study defines mechanisms required for ABC accumulation and function and highlights their responses to TLR7 as a contributer to sex bias in lupus.
Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118(5):1305–15. https://doi.org/10.1182/blood-2011-01-331462.
Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, Marrack P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest. 2017;127(4):1392–404. https://doi.org/10.1172/JCI91250.
Manni M, Gupta S, Ricker E, Chinenov Y, Park SH, Shi M, et al. Regulation of age-associated B cells by IRF5 in systemic autoimmunity. Nat Immunol. 2018;19(4):407–19. https://doi.org/10.1038/s41590-018-0056-8.
Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013;110(34):E3216–24. https://doi.org/10.1073/pnas.1312348110.
Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–304. https://doi.org/10.1182/blood-2011-01-330530.
Cancro MP, Age-Associated B. Cells. Annu Rev Immunol. 2020;38:315–40. https://doi.org/10.1146/annurev-immunol-092419-031130.
Phalke S, Marrack P. Age (autoimmunity) associated B cells (ABCs) and their relatives. Curr Opin Immunol. 2018;55:75–80. https://doi.org/10.1016/j.coi.2018.09.007.
Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49(4):725–39.e6. https://doi.org/10.1016/j.immuni.2018.08.015. This report identifies DN2 cells as a CD11c+T-bet+ B cell population that is expanded in SLE and is particularly responsive to TLR7 stimulation.
Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. 2018;9(1):1758. https://doi.org/10.1038/s41467-018-03750-7. Here, a population of CD11c+T-bet+ B cells is shown to accumulate in SLE patients and differentiate into autoantibody secreting plasma cells.
Arazi A, Rao DA, Berthier CC, Davidson A, Liu Y, Hoover PJ, et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol. 2019;20(7):902–14. https://doi.org/10.1038/s41590-019-0398-x. Single cell RNA-Seq defines multiple immune cell populations in lupus nephritis kidneys and indentifies relationships among them by developmental trajectory analysis.
Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol. 2015;195(1):71–9. https://doi.org/10.4049/jimmunol.1500055.
Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, et al. Cutting Edge: IL-4, IL-21, and IFN-gamma Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J Immunol. 2016;197(4):1023–8. https://doi.org/10.4049/jimmunol.1600522.
Zumaquero E, Stone SL, Scharer CD, Jenks SA, Nellore A, Mousseau B et al. IFNγ induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation. eLife. 2019;8:e41641. https://doi.org/10.7554/eLife.41641.
Zhou S, Li Q, Zhou S, Zhao M, Lu L, Wu H, et al. A novel humanized cutaneous lupus erythematosus mouse model mediated by IL-21-induced age-associated B cells. J Autoimmun. 2021;123: 102686. https://doi.org/10.1016/j.jaut.2021.102686.
Giltiay NV, Chappell CP, Sun X, Kolhatkar N, Teal TH, Wiedeman AE, et al. Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells. J Exp Med. 2013;210(12):2773–89. https://doi.org/10.1084/jem.20122798.
Wang T, Marken J, Chen J, Tran VB, Li QZ, Li M, et al. High TLR7 Expression Drives the Expansion of CD19(+)CD24(hi)CD38(hi) Transitional B Cells and Autoantibody Production in SLE Patients. Front Immunol. 2019;10:1243. https://doi.org/10.3389/fimmu.2019.01243.
Hamilton JA, Wu Q, Yang P, Luo B, Liu S, Hong H, et al. Cutting Edge: Endogenous IFN-beta Regulates Survival and Development of Transitional B Cells. J Immunol. 2017;199(8):2618–23. https://doi.org/10.4049/jimmunol.1700888.
Jacobs HM, Thouvenel CD, Leach S, Arkatkar T, Metzler G, Scharping NE, et al. Cutting Edge: BAFF Promotes Autoantibody Production via TACI-Dependent Activation of Transitional B Cells. J Immunol. 2016;196(9):3525–31. https://doi.org/10.4049/jimmunol.1600017.
Du SW, Jacobs HM, Arkatkar T, Rawlings DJ, Jackson SW. Integrated B Cell, Toll-like, and BAFF Receptor Signals Promote Autoantibody Production by Transitional B Cells. J Immunol. 2018;201(11):3258–68. https://doi.org/10.4049/jimmunol.1800393.
Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):816–25. https://doi.org/10.1038/sj.cdd.4401850.
Aue A, Szelinski F, Weissenberg SY, Wiedemann A, Rose T, Lino AC, et al. Elevated STAT1 expression but not phosphorylation in lupus B cells correlates with disease activity and increased plasmablast susceptibility. Rheumatology (Oxford). 2020;59(11):3435–42. https://doi.org/10.1093/rheumatology/keaa187.
Patel ZH, Lu X, Miller D, Forney CR, Lee J, Lynch A, et al. A plausibly causal functional lupus-associated risk variant in the STAT1-STAT4 locus. Hum Mol Genet. 2018;27(13):2392–404. https://doi.org/10.1093/hmg/ddy140.
Wu YY, Kumar R, Iida R, Bagavant H, Alarcon-Riquelme ME. BANK1 Regulates IgG Production in a Lupus Model by Controlling TLR7-Dependent STAT1 Activation. PLoS ONE. 2016;11(5): e0156302. https://doi.org/10.1371/journal.pone.0156302.
Xu W, Nair JS, Malhotra A, Zhang JJ. B cell antigen receptor signaling enhances IFN-gamma-induced Stat1 target gene expression through calcium mobilization and activation of multiple serine kinase pathways. J Interferon Cytokine Res. 2005;25(2):113–24. https://doi.org/10.1089/jir.2005.25.113.
Chodisetti SB, Fike AJ, Domeier PP, Schell SL, Mockus TE, Choi NM, et al. Serine Phosphorylation of the STAT1 Transactivation Domain Promotes Autoreactive B Cell and Systemic Autoimmunity Development. J Immunol. 2020;204(10):2641–50. https://doi.org/10.4049/jimmunol.2000170. This study finds that autoimmune B cell responses can be distinguished from responses to foreign antigen by a dependence of the former, but not the latter, on a single phosphoyrlation site in STAT1.
Kobayashi T, Shimabukuro-Demoto S, Yoshida-Sugitani R, Furuyama-Tanaka K, Karyu H, Sugiura Y, et al. The histidine transporter SLC15A4 coordinates mTOR-dependent inflammatory responses and pathogenic antibody production. Immunity. 2014;41(3):375–88. https://doi.org/10.1016/j.immuni.2014.08.011.
Thibault DL, Chu AD, Graham KL, Balboni I, Lee LY, Kohlmoos C, et al. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008;118(4):1417–26. https://doi.org/10.1172/JCI30065.
Xu W, Zhang JJ. Stat1-dependent synergistic activation of T-bet for IgG2a production during early stage of B cell activation. J Immunol. 2005;175(11):7419–24. https://doi.org/10.4049/jimmunol.175.11.7419.
Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99(8):5545–50. https://doi.org/10.1073/pnas.082114899.
Stone SL, Peel JN, Scharer CD, Risley CA, Chisolm DA, Schultz MD et al. T-bet Transcription Factor Promotes Antibody-Secreting Cell Differentiation by Limiting the Inflammatory Effects of IFN-gamma on B Cells. Immunity. 2019;50(5):1172–87 e7. https://doi.org/10.1016/j.immuni.2019.04.004.
Gomez Hernandez G, Morell M, Alarcon-Riquelme ME. The Role of BANK1 in B Cell Signaling and Disease. Cells. 2021;10(5). https://doi.org/10.3390/cells10051184.
Georg I, Diaz-Barreiro A, Morell M, Pey AL, Alarcon-Riquelme ME. BANK1 interacts with TRAF6 and MyD88 in innate immune signaling in B cells. Cell Mol Immunol. 2020;17(9):954–65. https://doi.org/10.1038/s41423-019-0254-9.
Jiang SH, Athanasopoulos V, Ellyard JI, Chuah A, Cappello J, Cook A, et al. Functional rare and low frequency variants in BLK and BANK1 contribute to human lupus. Nat Commun. 2019;10(1):2201. https://doi.org/10.1038/s41467-019-10242-9.
Ban T, Sato GR, Tamura T. Regulation and role of the transcription factor IRF5 in innate immune responses and systemic lupus erythematosus. Int Immunol. 2018;30(11):529–36. https://doi.org/10.1093/intimm/dxy032.
Pellerin A, Yasuda K, Cohen-Bucay A, Sandra V, Shukla P, Jr BKH et al. Monoallelic IRF5 deficiency in B cells prevents murine lupus. JCI Insight. 2021;6(15). https://doi.org/10.1172/jci.insight.141395.
De S, Zhang B, Shih T, Singh S, Winkler A, Donnelly R, et al. B Cell-Intrinsic Role for IRF5 in TLR9/BCR-Induced Human B Cell Activation, Proliferation, and Plasmablast Differentiation. Front Immunol. 2017;8:1938. https://doi.org/10.3389/fimmu.2017.01938.
Song S, De S, Nelson V, Chopra S, LaPan M, Kampta K, et al. Inhibition of IRF5 hyperactivation protects from lupus onset and severity. J Clin Invest. 2020;130(12):6700–17. https://doi.org/10.1172/JCI120288.
Li D, Matta B, Song S, Nelson V, Diggins K, Simpfendorfer KR et al. IRF5 genetic risk variants drive myeloid-specific IRF5 hyperactivation and presymptomatic SLE. JCI Insight. 2020;5(2). https://doi.org/10.1172/jci.insight.124020.
Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41(11):1234–7. https://doi.org/10.1038/ng.472.
Odhams CA, Roberts AL, Vester SK, Duarte CST, Beales CT, Clarke AJ, et al. Interferon inducible X-linked gene CXorf21 may contribute to sexual dimorphism in Systemic Lupus Erythematosus. Nat Commun. 2019;10(1):2164. https://doi.org/10.1038/s41467-019-10106-2.
Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107(46):19973–8. https://doi.org/10.1073/pnas.1014051107.
Kobayashi T, Nguyen-Tien D, Ohshima D, Karyu H, Shimabukuro-Demoto S, Yoshida-Sugitani R, et al. Human SLC15A4 is crucial for TLR-mediated type I interferon production and mitochondrial integrity. Int Immunol. 2021;33(7):399–406. https://doi.org/10.1093/intimm/dxab006.
Sasawatari S, Okamura T, Kasumi E, Tanaka-Furuyama K, Yanobu-Takanashi R, Shirasawa S, et al. The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice. Gastroenterology. 2011;140(5):1513–25. https://doi.org/10.1053/j.gastro.2011.01.041.
Katewa A, Suto E, Hui J, Heredia J, Liang J, Hackney J, et al. The peptide symporter SLC15a4 is essential for the development of systemic lupus erythematosus in murine models. PLoS ONE. 2021;16(1): e0244439. https://doi.org/10.1371/journal.pone.0244439.
Baccala R, Gonzalez-Quintial R, Blasius AL, Rimann I, Ozato K, Kono DH, et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc Natl Acad Sci U S A. 2013;110(8):2940–5. https://doi.org/10.1073/pnas.1222798110.
Heinz LX, Lee J, Kapoor U, Kartnig F, Sedlyarov V, Papakostas K, et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7-9. Nature. 2020;581(7808):316–22. https://doi.org/10.1038/s41586-020-2282-0. Several molecules encoded by SLE-susceptibility loci are linked in a biochemical pathway in this report.
Harris VM, Harley ITW, Kurien BT, Koelsch KA, Scofield RH. Lysosomal pH Is Regulated in a Sex Dependent Manner in Immune Cells Expressing CXorf21. Front Immunol. 2019;10:578. https://doi.org/10.3389/fimmu.2019.00578.
Harris VM, Koelsch KA, Kurien BT, Harley ITW, Wren JD, Harley JB, et al. Characterization of cxorf21 Provides Molecular Insight Into Female-Bias Immune Response in SLE Pathogenesis. Front Immunol. 2019;10:2160. https://doi.org/10.3389/fimmu.2019.02160.
Acharya M, Sokolovska A, Tam JM, Conway KL, Stefani C, Raso F, et al. alphav Integrins combine with LC3 and atg5 to regulate Toll-like receptor signalling in B cells. Nat Commun. 2016;7:10917. https://doi.org/10.1038/ncomms10917.
Raso F, Sagadiev S, Du S, Gage E, Arkatkar T, Metzler G, et al. alphav Integrins regulate germinal center B cell responses through noncanonical autophagy. J Clin Invest. 2018;128(9):4163–78. https://doi.org/10.1172/JCI99597.
Acharya M, Raso F, Sagadiev S, Gilbertson E, Kadavy L, Li QZ, et al. B Cell alphav Integrins Regulate TLR-Driven Autoimmunity. J Immunol. 2020;205(7):1810–8. https://doi.org/10.4049/jimmunol.1901056.
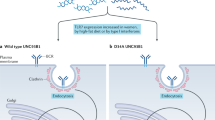
Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452(7184):234–8. https://doi.org/10.1038/nature06726.
Fukui R, Saitoh S, Kanno A, Onji M, Shibata T, Ito A, et al. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity. 2011;35(1):69–81. https://doi.org/10.1016/j.immuni.2011.05.010.
Fukui R, Saitoh S, Matsumoto F, Kozuka-Hata H, Oyama M, Tabeta K, et al. Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. J Exp Med. 2009;206(6):1339–50. https://doi.org/10.1084/jem.20082316.
Majer O, Liu B, Woo BJ, Kreuk LSM, Van Dis E, Barton GM. Release from UNC93B1 reinforces the compartmentalized activation of select TLRs. Nature. 2019;575(7782):371–4. https://doi.org/10.1038/s41586-019-1611-7.
Majer O, Liu B, Kreuk LSM, Krogan N, Barton GM. UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity. Nature. 2019;575(7782):366–70. https://doi.org/10.1038/s41586-019-1612-6. This study highlights the importance of proper TLR7 trafficking for the termination of signaling and the prevention of autoimmunity.
Kolhatkar NS, Scharping NE, Sullivan JM, Jacobs HM, Schwartz MA, Khim S, et al. B-cell intrinsic TLR7 signals promote depletion of the marginal zone in a murine model of Wiskott-Aldrich syndrome. Eur J Immunol. 2015;45(10):2773–9. https://doi.org/10.1002/eji.201545644.
Tohme M, Maisonneuve L, Achour K, Dussiot M, Maschalidi S, Manoury B. TLR7 trafficking and signaling in B cells is regulated by the MHCII-associated invariant chain. J Cell Sci. 2020;133(5). https://doi.org/10.1242/jcs.236711.
Mullard A. FDA approves AstraZeneca’s anifrolumab for lupus. Nat Rev Drug Discov. 2021;20(9):658. https://doi.org/10.1038/d41573-021-00139-y.
Boedigheimer MJ, Martin DA, Amoura Z, Sanchez-Guerrero J, Romero-Diaz J, Kivitz A, et al. Safety, pharmacokinetics and pharmacodynamics of AMG 811, an anti-interferon-gamma monoclonal antibody, in SLE subjects without or with lupus nephritis. Lupus Sci Med. 2017;4(1): e000226. https://doi.org/10.1136/lupus-2017-000226.
Bocharnikov AV, Keegan J, Wacleche VS, Cao Y, Fonseka CY, Wang G et al. PD-1hiCXCR5- T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight. 2019;4(20). https://doi.org/10.1172/jci.insight.130062.
Ren HM, Lukacher AE, Rahman ZSM, Olsen NJ. New developments implicating IL-21 in autoimmune disease. J Autoimmun. 2021;122: 102689. https://doi.org/10.1016/j.jaut.2021.102689.
Chaichian Y, Wallace DJ. Innovative Trials and New Opportunities in SLE. Rheum Dis Clin North Am. 2021;47(3):481–99. https://doi.org/10.1016/j.rdc.2021.04.010.
Kolios AGA, Yoshida N, Tsokos GC. New therapeutic approaches in systemic lupus erythematosus. Curr Opin Rheumatol. 2021;33(2):181–9. https://doi.org/10.1097/BOR.0000000000000772.
Liossis SN, Staveri C. What’s New in the Treatment of Systemic Lupus Erythematosus. Front Med (Lausanne). 2021;8: 655100. https://doi.org/10.3389/fmed.2021.655100.
Mok CC. The Jakinibs in systemic lupus erythematosus: progress and prospects. Expert Opin Investig Drugs. 2019;28(1):85–92. https://doi.org/10.1080/13543784.2019.1551358.
Celhar T, Lu HK, Benso L, Rakhilina L, Lee HY, Tripathi S, et al. TLR7 Protein Expression in Mild and Severe Lupus-Prone Models Is Regulated in a Leukocyte, Genetic, and IRAK4 Dependent Manner. Front Immunol. 2019;10:1546. https://doi.org/10.3389/fimmu.2019.01546.
Corzo CA, Varfolomeev E, Setiadi AF, Francis R, Klabunde S, Senger K et al. The kinase IRAK4 promotes endosomal TLR and immune complex signaling in B cells and plasmacytoid dendritic cells. Sci Signal. 2020;13(634). https://doi.org/10.1126/scisignal.aaz1053.
Dudhgaonkar S, Ranade S, Nagar J, Subramani S, Prasad DS, Karunanithi P, et al. Selective IRAK4 Inhibition Attenuates Disease in Murine Lupus Models and Demonstrates Steroid Sparing Activity. J Immunol. 2017;198(3):1308–19. https://doi.org/10.4049/jimmunol.1600583.
Murphy M, Pattabiraman G, Manavalan TT, Medvedev AE. Deficiency in IRAK4 activity attenuates manifestations of murine Lupus. Eur J Immunol. 2017;47(5):880–91. https://doi.org/10.1002/eji.201646641.
Nanda SK, Lopez-Pelaez M, Arthur JS, Marchesi F, Cohen P. Suppression of IRAK1 or IRAK4 Catalytic Activity, but Not Type 1 IFN Signaling, Prevents Lupus Nephritis in Mice Expressing a Ubiquitin Binding-Defective Mutant of ABIN1. J Immunol. 2016;197(11):4266–73. :https://doi.org/10.4049/jimmunol.1600788.
Winkler A, Sun W, De S, Jiao A, Nusrat Sharif M, Symanowicz PT, et al. The IRAK4 kinase inhibitor PF-06650833 blocks inflammation in preclinical models of rheumatologic disease and in humans enrolled in a randomized clinical trial. Arthritis Rheumatol. 2021. https://doi.org/10.1002/art.41953.
Hjorton K, Hagberg N, Israelsson E, Jinton L, Berggren O, Sandling JK, et al. Cytokine production by activated plasmacytoid dendritic cells and natural killer cells is suppressed by an IRAK4 inhibitor. Arthritis Res Ther. 2018;20(1):238. https://doi.org/10.1186/s13075-018-1702-0.
Ban T, Kikuchi M, Sato GR, Manabe A, Tagata N, Harita K, et al. Genetic and chemical inhibition of IRF5 suppresses pre-existing mouse lupus-like disease. Nat Commun. 2021;12(1):4379. https://doi.org/10.1038/s41467-021-24609-4.
Dong X, Antao OQ, Song W, Sanchez GM, Zembrzuski K, Koumpouras F, et al. Type I Interferon-Activated STAT4 Regulation of Follicular Helper T Cell-Dependent Cytokine and Immunoglobulin Production in Lupus. Arthritis Rheumatol. 2021;73(3):478–89. https://doi.org/10.1002/art.41532.
Funding
A.S. is supported by NIH grants AI122720, AI161307, and AR072598. She is a Southwestern Medical Foundation Scholar in Biomedical Research and holds the Peggy Chavellier Professorship in Arthritis Research and Treatment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
A.S. holds stock in Amgen.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Systemic Lupus Erythematosus
Rights and permissions
About this article
Cite this article
Satterthwaite, A.B. TLR7 Signaling in Lupus B Cells: New Insights into Synergizing Factors and Downstream Signals. Curr Rheumatol Rep 23, 80 (2021). https://doi.org/10.1007/s11926-021-01047-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-01047-1