Abstract
Purpose of Review
To describe the contributions of osteocytes to the lesions in Paget’s disease, which are characterized by locally overactive bone resorption and formation.
Recent Findings
Osteocytes, the most abundant cells in bone, are altered in Paget’s disease lesions, displaying increased size, decreased canalicular length, incomplete differentiation, and less sclerostin expression compared to controls in both patients and mouse models. Pagetic lesions show increased senescent osteocytes that express RANK ligand, which drives osteoclastic bone resorption. Abnormal osteoclasts in Paget’s disease secrete abundant IGF1, which enhances osteocyte senescence, contributing to lesion formation.
Summary
Recent data suggest that osteocytes contribute to lesion formation in Paget’s disease by responding to high local IGF1 released from abnormal osteoclasts. Here we describe the characteristics of osteocytes in Paget’s disease and their role in bone lesion formation based on recent results with mouse models and supported by patient data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sir James Paget, surgeon to Queen Victoria, described a disease of bone in 1877 and an unrelated disease of the breast earlier. Both disorders still bear his name. Paget’s disease of bone is characterized by localized areas of increased bone resorption coupled with exuberant new bone formation and with high circulating alkaline phosphatase activity. Paget’s disease occurs mostly in the elderly. The lesions are focal and solitary and do not spread. Both genetic and viral etiologies contribute to the phenotype of what is now known to be a spectrum of bone disorders [1•, 2].
The primary visible cellular abnormality in Paget’s disease resides in the osteoclasts, which are increased in size and multinucleation compared to normal osteoclasts. They secrete more interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF1) and are hyperresponsive to 1,25-dihydroxyvitamin D3 [3,4,5]. The osteoclast as the primary site of cellular abnormality is supported by the efficacy of treatment with bisphosphonate inhibitors. A single infusion of zoledronic acid results in multi-year elimination of pagetic lesions, often for the life of the patient. Infrequent recurrences are at the original site [6]. Since osteoclasts arise from precursors that circulate in peripheral blood, how do pagetic lesions remain focal? This conundrum suggests that pagetic bone differs from uninvolved skeletal sites in the same patient and that the differences maintain the local persistence of the lesion. Osteocytes are immobile, long-lived, regulate osteoblast and osteoclast functions, and are subject to epigenetic reprogramming [7•], making them prime candidates to be central regulators of pagetic lesions.
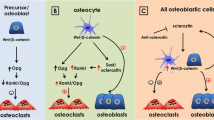
We hypothesize that osteocytes play a key role in pagetic bone lesions. Figure 1A cartoons how paracrine interactions between pagetic osteoclasts and adjacent osteocytes might sustain the abnormal phenotype of pagetic bone. Pagetic osteoclasts secrete IGF1, which increases senescence of nearby osteocytes; these cells in turn secrete more receptor activator of nuclear factor kappa beta ligand (RANKL) and less sclerostin: the former resulting in more osteoclastic osteolysis, and the latter permitting more osteoblastic new bone formation. In what follows, we review the genetic and environmental conditions that can contribute to Paget’s disease and then focus on experiments with transgenic mice where measles virus nucleocapsid protein (MVNP) is targeted to the osteoclast lineage. Around 1 year of age, these mice develop bone lesions that resemble those seen in patients with Paget’s disease.
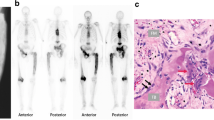
A Proposed role of osteocytes in pagetic bone lesions. Osteoclast-IGF1 induces osteocyte senescence and RANKL production, suppresses osteocyte sclerostin, and enhances the formation of pagetic osteoclasts, leading to increased local bone destruction coupled with disorganized new bone formation (from [23••]). B Images of osteocytes from a Paget’s disease patient and a TRAP-MVNP mouse. Spinal bone sections were treated with Ploton silver to stain the canalicular and cement lines. The Paget’s patient was a 59-year-old male, and the normal subject was a 58-year-old male; both bone samples were from the spine (provided by Dr. Brendan Boyce, University of Rochester). TRAP-MVNP and WT mouse sections were taken 500 µm below the distal growth plate of 20-month-old femurs. Scale bars are 10 µm. Images are of different sections from the samples published in Miyagawa et al. [23••]
Both genetic and environmental factors can cause abnormal osteoclast activity in Paget’s disease, recently reviewed by Gennari et al. [8•]. Genes where mutations trigger Paget’s disease lesions include sequestosome 1 (p62), ZNF687, and profilin1. Mutations in other genes, such as optineurin and RANK, predispose to the development of pagetic bone lesions but are insufficient to cause them. Triggering mutations and environmental factors have been confirmed in mouse models, although Paget’s disease lesions are usually found in mice at >1 year of age, making such animal experiments slow and expensive. As is characteristic of the human disease, Paget’s disease in mice is responsive to treatment with bisphosphonate anti-resorptive agents [9].
Over 21 mutations in human SQSTM1/p62 (a scaffold protein with a key role in autophagy) are linked to familial Paget’s disease (about 30% of all Paget’s disease cases), with p62P392L the most frequent [10•, 11, 12]. We found that osteoclast precursors expressing p62P392L did not form bone lesions and lacked the osteoclastic characteristics of Paget’s disease [10•]. Knock-in mice carrying the murine equivalent of human p62P392L (p62KI mice) had increased osteoclast precursors but histologically normal bones [13]. Daroszewska and co-workers [11] reported bone lesions in a similar p62KI mouse, but these were atypical of Paget’s disease. The results suggest that additional factors contribute to the development of Paget’s disease due to p62 mutation [14]. Pagetic bone lesions also occur in a variety of complex genetic disorders, such as those involving profilin 1 and valosin-containing protein/p97 [15, 16], but these multisystem proteinopathies are early onset and have extra-skeletal symptoms along with multiple bone lesions, unlike the late onset, solitary presentation of common Paget’s disease [17].
Environmental factors, including infections by paramyxoviruses (e.g., measles virus or canine distemper virus), have been implicated in the pathogenesis of Paget’s disease [1•] since 1976, when Miller and Singer described viral nuclear inclusions in pagetic osteoclasts [8•]. Despite the course of nearly 50 years and more than 100 publications on measles virus in Paget’s disease, a viral contribution to clinical Paget’s disease remains controversial. Some groups have detected viral inclusions and paramyxoviral transcripts and antigens in the osteoclasts of pagetic lesions [18, 19], while others have exhaustively failed to do so [20]. Part of the challenge is explaining how abnormalities in osteoclast morphology and function could appear in an elderly patient with Paget’s disease decades after a viral infection that is no longer detectable.
Precursor cells differentiate into pagetic osteoclasts in vitro when manipulated to express MVNP protein (which in measles-infected cells has transcriptional regulatory activity) [12, 21••]. When an MVNP transgene was targeted to the osteoclast using the tartrate-resistant acid phosphatase (TRAP) promoter, 43% of 16–20-month-old mice developed bone lesions characteristic of Paget’s disease, with abnormal osteoclasts and irregular, jigsaw-puzzle shaped bone particles that are hallmarks of Paget’s disease [21••, 22, 23••]. Interestingly, marrow cultures of osteoclasts from Paget’s patients carrying the p62P392L mutation formed pagetic osteoclasts in vitro only if they co-expressed MVNP. Pagetic differentiation was blocked by antisense RNA to MVNP [10•]. When p62KI mice were bred to TRAP-MVNP mice, the p62KI/MVNP mice developed greater numbers of pagetic osteoclasts with age and bone lesions that were strikingly like those seen in patients [10•]. MVNP-transgenic osteoclasts secrete abundant IGF1 and interleukin-6 (IL-6) [14]. In MVNP mice, deletion of IL-6 [5] or conditional knockout of IGF1 in the osteoclast lineage abrogated formation of pagetic osteoclasts and bone lesions in vivo [22].
Roles of IL-6 and IGF1 in Paget’s Disease
Paget’s disease patients have elevated IL-6 in marrow plasma and peripheral blood [3], and their osteoclasts express high IL-6 [24]. MVNP induces high IL-6 expression via downregulation of FoxO3/Sirt1 signaling, increasing the formation of pagetic osteoclasts and bone lesions [24]. IL-6 ablation in MVNP mice blocked development of pagetic osteoclasts and bone lesions in vivo [5]. However, transgenic overexpression of IL-6 in osteoclasts did not induce a pagetic phenotype in osteoclasts or pagetic lesions in mice [5], suggesting that other factors induced by MVNP are needed for the development of Paget’s disease. IL-6 can stimulate the differentiation of primary calvarial osteoblasts and induce RANKL in osteoblastic precursors [25], but its overexpression in mice reduces osteoblast numbers, and it may inhibit bone formation in vivo. Wu et al. reported [26] that IL-6 enhanced osteocyte-mediated osteoclast formation by increasing RANKL. Thus, increased IL-6 from pagetic osteoclasts could enhance RANKL production by osteocytes to further increase local osteoclast formation. Immunohistochemical studies of bones from Paget’s disease patients showed that pagetic osteoclasts contained abundant platelet-derived growth factor, transforming growth factor-β and IGF1, as well as IL-6 [27]. Gene expression profiling studies of highly purified cells from MVNP, p62KI, and wild type (WT) mice showed that MVNP-expressing osteoclasts contained large amounts of IGF1 mRNA, while osteoclasts from WT and p62KI mice did not [14]. Importantly, conditional knockout of IGF1 in osteoclasts blocked the development of pagetic bone lesions and restricted osteocyte differentiation in MVNP mice [22].
Characteristics of Osteocytes in Paget’s Disease
Osteoclasts are hyper-multinucleated and increased in number in pagetic bone lesions [10•, 28]. The lesions are solitary and persist for years, while active osteoclasts are short-lived derivatives from circulating precursors. The persistent memory component of Paget’s lesions could be provided by long-lived osteocytes resident within bone [29]. Unusual canalicular bone resorption around osteocytes in Paget’s disease patients was reported as early as 1968 [30], but this is not unique to Paget’s disease. Ultrastructural changes were later reported in pagetic osteocytes [31]. Viral inclusions and sequences have been detected repeatedly in pagetic osteoclasts, but paramyxoviral transcripts without viral inclusions have been found only infrequently in osteoblasts and osteocytes. The photomicrographs in Fig. 1B, like previously published images [21••, 22, 23••], show Ploton silver staining of bones from a Paget’s disease patient, an MVNP mouse, a normal donor, and a WT mouse. The canalicular lengths of osteocytes in MVNP mice were shorter than in WT mice but did not differ between sexes [23••].
The osteocytes in a bone biopsy from a patient with Paget’s disease showed reduced sclerostin staining compared to a control bone biopsy from a normal patient [23••]. Sclerostin expression and dendritic processes of the osteocytes in MVNP mice were significantly reduced, compared to WT mice, in regions of bone that lacked pagetic lesions. Osteocyte sclerostin expression and dendritic processes were further reduced in MVNP mice at sites of pagetic bone lesions, which also had lower numbers of sclerostin-expressing osteocytes per bone area and decreased canalicular length compared with MVNP mice without pagetic bone lesions or WT mice. Circulating serum sclerostin concentrations were similar in all WT and MVNP mice [23••], perhaps because the osteocytes in the pagetic bone lesions are a very low percentage of all the sclerostin-secreting osteocytes in the mice. At least five papers report circulating levels of sclerostin in Paget’s disease patients, with two suggesting it is increased, while three found no change [32,33,34].
Osteocyte differentiation is an active process [35]. These cells develop from a polygonal osteoblastic precursor to one with cytoplasmic extensions directed toward the mineralizing front and that can extend to the bone surface to interact with other cells, bone marrow cells, and the vascular space [36]. Osteocyte differentiation occurs as precursors become embedded in osteoid and begin to extend dendritic processes, followed by expression of DMP1 and CapG, proteins which regulate the cytoskeleton. Mature osteocytes within mineralized bone secrete sclerostin, FGF23, and ORP150, a factor thought to protect the cells from hypoxia [37, 38].
Sclerostin secretion and dendrite formation are characteristics of mature osteocytes [39], which can also make IGF1 [7•], while IGF1 enhances osteocyte differentiation stimulated by parathyroid hormone [40]. We analyzed mRNAs in primary osteocytes isolated by collagenase digestion of long bones from 20-month-old WT and MVNP mice. Sclerostin (Sost) mRNA in cells from MVNP mice was reduced by 30% compared to WT, while Igf1 mRNA was unchanged [23••]. Fluorescent immunostaining of osteocytes from MVNP mice showed decreased average intensity of staining for the maturation markers DMP1 and sclerostin, compared to WT mice [23••].
Only a limited number of osteocytes can be obtained by collagenase digestion of mouse bones, so we studied osteoblasts and osteocytes from cells that grow out of bones in tissue culture after removal from WT and MVNP mice [23••]. Future experiments would assess local sclerostin expression by osteocytes within histological sections from pagetic patients’ bone lesions compared to controls to determine if osteocyte maturation is impaired or delayed in Paget’s disease.
Senescence and Osteocytes
Farr and colleagues recently characterized senescent osteocytes using highly purified cells from 6- and 24-month-old mice. Old mice showed a 6-fold increase in senescent osteocytes (11% vs. 2%), which displayed a senescence-associated secretory phenotype with high expression of 23 genes, including IL-6, IL-1, NF-κB, GM-CSF, IL-8, MCP1, and M-CSF, a gene signature with pro-osteoclastogenic potential [41••]. Upregulation of p16INK4A, telomere shortening, and decondensation of pericentromeric satellite DNA marked the senescent cells. Tran et al. [42] reported that prolonged exposure of mouse and human fibroblasts to IGF1 induced premature cellular senescence. IGF1 inhibited SIRT1 deacetylase activity, resulting in increased p53 acetylation, stabilization, and activation, leading to premature senescence. We hypothesize that a local subset of osteoclasts in Paget’s disease patients may undergo senescence due to exposure to high local levels of osteoclast-IGF1, forming a niche that activates precursors to increase pagetic-OCL formation. Mutations in p62 could enhance pagetic osteoclast activation of senescent osteocytes through increased NF-κB signaling [43].
Senescent Osteocytes are Important Sources of RANKL in Paget’s Disease
Senescent osteocytes express RANKL, which contributes to the cortical bone loss that occurs with age [44••]. Yan and colleagues recently identified an intronic enhancer of RANKL that may be selectively activated in osteocytic cells and could be stimulated by senescence [45•], suggesting a molecular basis for RANKL regulation in osteocytes, with therapeutic implications for various skeletal disorders.
The regulation of osteocyte RANKL production during senescence [43, 44••, 45•] was examined by comparing p16INK4A and RANKL in MVNP and WT mice by immunofluorescent staining of single cells. Both p16INK4A and RANKL were more highly expressed in MVNP than in WT mice (preliminary data, not shown). p16INK4A- and RANKL-expressing osteocytes in MVNP mice were increased 2.5- to 3.5-fold compared with WT. Moreover, p16INK4A/RANKL double-positive osteocytes accounted for 25% of total osteocytes in MVNP mice, 3-fold higher than in WT [23••]. The results confirm previous observations that osteocyte RANKL production is part of a senescence-associated secretory phenotype. Twenty-five percent of osteocytes in pagetic bone lesions showed senescence markers and secreted RANKL [23••]. These phenotypic changes in osteocytes may be regulated by IGF1 signaling, which is known to induce intracellular oxidative burden and associated oxidative damage and can lead to premature senescence. Osteoclast-secreted IGF1 in Paget’s disease could thus act on nearby osteocytes to induce senescence and increase local RANKL.
Conclusions
Paget’s disease of bone is characterized by skeletal sites of persistent high bone formation coupled to high bone resorption with a primary abnormality of osteoclasts. Such lesions do not spread, and the osteocytes in them are now known to be abnormal both morphologically and biochemically. They display altered canalicular processes, express increased RANKL, which may contribute to increased osteolysis, and secrete less sclerostin, which could permit increased new bone formation. High local concentrations of IGF1 secreted from pagetic osteoclasts increase markers of osteocyte senescence; secretion of RANKL is induced from senescent osteocytes and contributes to the maintenance of pagetic bone lesions. It is presently uncertain whether osteocytes also play a major role in the coupling between osteoclastic bone destruction and osteoblastic new bone formation characteristic of Paget’s disease of bone.
Future Directions
The local elevation of IGF1 in pagetic lesions appears to act by induction of osteocyte senescence. It is currently unknown whether elevated osteoclast-IGF1 and osteocyte senescence are central factors in the development of pagetic lesions in Paget’s disease due to causes such as mutations in p62 or non-measles environmental factors. In future studies, pagetic bone samples from patients with Paget’s disease and from mouse models could be stained for osteocyte morphology and expression of RANKL, sclerostin and senescence markers and for IGF1 in osteoclasts.
Bone formation remains coupled to resorption in pagetic lesions. This is in part due to the expression of the coupling factors EphB2 on osteoclasts and EphB4 on osteoblasts, but may also be regulated by osteocytes, making this another area for future study. It remains unclear what initiates pagetic lesions. Since Paget’s disease occurs focally and late in life, it could be the result of a stochastic event at the site of a future lesion, perhaps involving a local cluster of senescent osteocytes which attract osteoclast precursors. This question could be addressed by homing experiments with transgenic mice and genetically marked osteoclast precursor cells.
Paget’s disease of bone is efficiently treated with anti-resorptive agents. Bisphosphonates not only kill osteoclasts but increase osteocyte viability, which could contribute to their efficacy against Paget’s, offering another area for future study. The altered phenotype of osteocytes within persistent pagetic lesions suggests that the cells have altered gene expression that could be due to epigenetic reprogramming, yet another area for future investigation. Although now an easily treated skeletal disorder, Paget’s disease of bone continues to provide insights into the cellular regulation of bone turnover and to suggest new roles for osteocytes as central controllers of osteoclast and osteoblast actions and interactions.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Singer FR. Paget’s disease of bone. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. An excellent review of the history and clinical aspects of Paget’s disease of bone.
Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115(2):200–8. https://doi.org/10.1172/jci24281.
Roodman GD, Kurihara N, Ohsaki Y, Kukita A, Hosking D, Demulder A, Smith JF, Singer FR. Interleukin 6. A potential autocrine/paracrine factor in Paget’s disease of bone. J Clin Invest. 1992;89(1):46–52. https://doi.org/10.1172/JCI115584.
Galson DL, Roodman GD. Pathobiology of Paget’s disease of bone. J Bone Metab. 2014;21(2):85–98. https://doi.org/10.11005/jbm.2014.21.2.85.
Teramachi J, Zhou H, Subler MA, Kitagawa Y, Galson DL, Dempster DW, Windle JJ, Kurihara N, Roodman GD. Increased IL-6 expression in osteoclasts is necessary but not sufficient for the development of Paget’s disease of bone. J Bone Miner Res. 2014;29(6):1456–65. https://doi.org/10.1002/jbmr.2158.
Cundy T, Maslowski K, Grey A, Reid IR. Durability of response to zoledronate treatment and competing mortality in Paget’s disease of bone. J Bone Miner Res. 2017;32(4):753–6. https://doi.org/10.1002/jbmr.3029.
• Delgado-Calle J, Bellido T. The osteocyte as a signaling cell. Physiol Rev. 2022;102(1):379–410. https://doi.org/10.1152/physrev.00043.2020. Up-to-date review of osteocyte signaling, which provides major contributions to Paget’s disease of bone.
• Gennari L, Rendina D, Merlotti D, Cavati G, Mingiano C, Cosso R, Materozzi M, Pirrotta F, Abate V, Calabrese M, Falchetti A. Update on the pathogenesis and genetics of Paget’s disease of bone. Front Cell Dev Biol. 2022;10:932065. https://doi.org/10.3389/fcell.2022.932065. A clear recent summary of environmental and genetic causes of Paget’s disease of bone.
Ling Z, Aini H, Kajikawa S, Shirakawa J, Tsuji K, Asou Y, Koga H, Sekiya I, Nifuji A, Noda M, Ezura Y. Osteolytic bone loss and skeletal deformities in a mouse model for early-onset Paget’s disease of bone with PFN1 mutation are treatable by alendronate. Pharmaceuticals (Basel). 2023;16(10):1395. https://doi.org/10.3390/ph16101395.
• Kurihara N, Hiruma Y, Yamana K, Michou L, Rousseau C, Morissette J, Galson DL, Teramachi J, Zhou H, Dempster DW, Windle JJ, Brown JP, Roodman GD. Contributions of the measles virus nucleocapsid gene and the SQSTM1/p62(P392L) mutation to Paget’s disease. Cell Metab. 2011;13(1):23–34. https://doi.org/10.1016/j.cmet.2010.12.002. An important demonstration of MVNP and p62 effects on mouse models of Paget’s bone disease.
Daroszewska A, van Hof RJ, Rojas JA, Layfield R, Landao-Basonga E, Rose L, Rose K, Ralston SH. A point mutation in the ubiquitin-associated domain of SQSMT1 is sufficient to cause a Paget’s disease-like disorder in mice. Hum Mol Genet. 2011;20(14):2734–44. https://doi.org/10.1093/hmg/ddr172.
Gallone S, Di Stefano M, Rainero I, Fenoglio P, Gravante E, Incardona S, Acutis PL, Maniaci MG, Isaia GC, Pinessi L. Detection of exon 8 mutations in sqstm1/p62 gene by mutation-specific restriction enzyme digestion: a sensitive screening for Paget disease of bone. Panminerva Med. 2011;53(1):71–2.
Menaa C, Reddy SV, Kurihara N, Maeda H, Anderson D, Cundy T, Cornish J, Singer FR, Bruder JM, Roodman GD. Enhanced RANK ligand expression and responsivity of bone marrow cells in Paget’s disease of bone. J Clin Invest. 2000;105(12):1833–8. https://doi.org/10.1172/jci9133.
Teramachi J, Nagata Y, Mohammad K, Inagaki Y, Ohata Y, Guise T, Michou L, Brown JP, Windle JJ, Kurihara N, Roodman GD. Measles virus nucleocapsid protein increases osteoblast differentiation in Paget’s disease. J Clin Invest. 2016;126(3):1012–22. https://doi.org/10.1172/JCI82012.
Boock V, Roy B, Pfeffer G, Kimonis V. Therapeutic developments for valosin-containing protein mediated multisystem proteinopathy. Curr Opin Neurol. 2023;36(5):432–40. https://doi.org/10.1097/WCO.0000000000001184.
Huybrechts Y, De Ridder R, Steenackers E, Devogelaer JP, Mortier G, Hendrickx G, Van Hul W. Genetic screening of ZNF687 and PFN1 in a Paget’s disease of bone cohort indicates an important role for the nuclear localization signal of ZNF687. Calcif Tissue Int. 2023;113(5):552–7. https://doi.org/10.1007/s00223-023-01137-5.
Siris ES, Roodman GD. Paget’s disease of bone. In: Rosen CJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. Washington DC: American Society for Bone and Mineral Research: Wiley-Blackwell; 2013.659-68.
Harvey L, Gray T, Beneton MN, Douglas DL, Kanis JA, Russell RG. Ultrastructural features of the osteoclasts from Paget’s disease of bone in relation to a viral aetiology. J Clin Pathol. 1982;35(7):771–9. https://doi.org/10.1136/jcp.35.7.771.
Basle MF, Rebel A, Fournier JG, Russell WC, Malkani K. On the trail of paramyxoviruses in Paget’s disease of bone. Clin Orthop Relat Res. 1987;217:9–15.
Helfrich MH, Hobson RP, Grabowski PS, Zurbriggen A, Cosby SL, Dickson GR, Fraser WD, Ooi CG, Selby PL, Crisp AJ, Wallace RG, Kahn S, Ralston SH. A negative search for a paramyxoviral etiology of Paget’s disease of bone: molecular, immunological, and ultrastructural studies in UK patients. J Bone Miner Res. 2000;15(12):2315–29. https://doi.org/10.1359/jbmr.2000.15.12.2315.
•• Kurihara N, Zhou H, Reddy SV, Garcia Palacios V, Subler MA, Dempster DW, Windle JJ, Roodman GD. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget’s disease-like bone lesions in mice. J Bone Miner Res. 2006;21(3):446–55. https://doi.org/10.1359/JBMR.051108. First demonstration that MVNP targeted to osteoclasts could induce Paget’s disease of bone in mice.
Miyagawa K, Ohata Y, Delgado-Calle J, Teramachi J, Zhou H, Dempster DD, Subler MA, Windle JJ, Chirgwin JM, Roodman GD, Kurihara N. Osteoclast-derived IGF1 is required for pagetic lesion formation in vivo. JCI Insight. 2020;5(6):e133113. https://doi.org/10.1172/jci.insight.133113.
•• Miyagawa K, Tenshin H, Mulcrone PL, Delgado-Calle J, Subler MA, Windle JJ, Chirgwin JM, Roodman GD, Kurihara N. Osteoclast-derived IGF1 induces RANKL production in osteocytes and contributes to pagetic lesion formation. JCI Insight. 2023;8(14):e159838. https://doi.org/10.1172/jci.insight.159838. Demonstration that osteoclast-derived IGF1 acts on osteocytes, contributing to pagetic lesions in mice.
Wang FM, Sarmasik A, Hiruma Y, Sun Q, Sammut B, Windle JJ, Roodman GD, Galson DL. Measles virus nucleocapsid protein, a key contributor to Paget’s disease, increases IL-6 expression via down-regulation of FoxO3/Sirt1 signaling. Bone. 2013;53(1):269–76. https://doi.org/10.1016/j.bone.2012.12.007.
Bellido T, Borba VZ, Roberson P, Manolagas SC. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997;138(9):3666–76. https://doi.org/10.1210/endo.138.9.5364.
Wu Q, Zhou X, Huang D, Ji Y, Kang F. IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell Physiol Biochem. 2017;41(4):1360–9. https://doi.org/10.1159/000465455.
Mills BG, Frausto A. Cytokines expressed in multinucleated cells: Paget’s disease and giant cell tumors versus normal bone. Calcif Tissue Int. 1997;61(1):16–21. https://doi.org/10.1007/s002239900285.
Neale SD, Smith R, Wass JA, Athanasou NA. Osteoclast differentiation from circulating mononuclear precursors in Paget’s disease is hypersensitive to 1,25-dihydroxyvitamin D3 and RANKL. Bone. 2000;27(3):409–16. https://doi.org/10.1016/s8756-3282(00)00345-8.
Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421746. https://doi.org/10.1155/2015/421746.
Belanger LF, Jarry L, Uhthoff HK. Osteocytic osteolysis in Paget’s disease. Rev Can Biol. 1968;27(1):37–44.
Singer FR, Mills BG, Gruber HE, Windle JJ, Roodman GD. Ultrastructure of bone cells in Paget’s disease of bone. J Bone Miner Res. 2006;21(Suppl 2):51–4. https://doi.org/10.1359/jbmr.06s209.
Yavropoulou MP, van Lierop AH, Hamdy NA, Rizzoli R, Papapoulos SE. Serum sclerostin levels in Paget’s disease and prostate cancer with bone metastases with a wide range of bone turnover. Bone. 2012;51(1):153–7. https://doi.org/10.1016/j.bone.2012.04.016.
Idolazzi L, Fassio A, Tripi G, Braga V, Viapiana O, Adami G, Rossini M, Gatti D. Circulating Dickkopf-1 and sclerostin in patients with Paget’s disease of bone. Clin Rheumatol. 2017;36(4):925–8. https://doi.org/10.1007/s10067-016-3497-1.
Chen L, Gao G, Shen L, Yue H, Zhang G, Zhang Z. Serum sclerostin and its association with bone turnover marker in metabolic bone diseases. Dis Markers. 2022;2022:7902046. https://doi.org/10.1155/2022/7902046.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. https://doi.org/10.1002/jbmr.320.
Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, Yoneda T, Mohammad KS, Plotkin LI, Roodman GD, Bellido T. Bidirectional Notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76(5):1089–100. https://doi.org/10.1158/0008-5472.CAN-15-1703.
Montesi M, Jahn K, Bonewald L, Stea S, Bordini B, Beraudi A. Hypoxia mediates osteocyte ORP150 expression and cell death in vitro. Mol Med Rep. 2016;14(5):4248–54. https://doi.org/10.3892/mmr.2016.5790.
Guo D, Keightley A, Guthrie J, Veno PA, Harris SE, Bonewald LF. Identification of osteocyte-selective proteins. Proteomics. 2010;10(20):3688–98. https://doi.org/10.1002/pmic.201000306.
Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506. https://doi.org/10.1146/annurev-physiol-021119-034332.
Qiu T, Crane JL, Xie L, Xian L, Xie H, Cao X. IGF-I induced phosphorylation of PTH receptor enhances osteoblast to osteocyte transition. Bone Res. 2018;6:5. https://doi.org/10.1038/s41413-017-0002-7.
•• Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S. Identification of senescent cells in the bone microenvironment. J Bone Miner Res. 2016;31(11):1920–9. https://doi.org/10.1002/jbmr.2892. Detailed characterization of senescence in bone cells including osteocytes.
Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao ZX. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13(4):669–78. https://doi.org/10.1111/acel.12219.
McManus S, Roux S. The adaptor protein p62/SQSTM1 in osteoclast signaling pathways. J Mol Signal. 2012;7:1. https://doi.org/10.1186/1750-2187-7-1.
•• Kim HN, Xiong J, MacLeod RS, Iyer S, Fujiwara Y, Cawley KM, Han L, He Y, Thostenson JD, Ferreira E, Jilka RL, Zhou D, Almeida M, O’Brien CA. Osteocyte RANKL is required for cortical bone loss with age and is induced by senescence. JCI Insight. 2020;5(19):e138815. https://doi.org/10.1172/jci.insight.138815. Important insight into RANKL expression in senescent osteocytes.
• Yan M, Tsukasaki M, Muro R, Ando Y, Nakamura K, Komatsu N, Nitta T, Okamura T, Okamoto K, Takayanagi H. Identification of an intronic enhancer regulating RANKL expression in osteocytic cells. Bone Res. 2023;11(1):43. https://doi.org/10.1038/s41413-023-00277-6. Identification of a transcriptional mechanism for regulation of RANKL in osteocytes.
Funding
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS): NIH R01-AR058308 (GDR) and R01-AR090116-01 (NK). Services in support of this research project were provided by the Virginia Commonwealth University Massey Cancer Center Transgenic/Knockout Mouse Core, supported in part with funding from NIH-NCI Cancer Center Support grant P30 CA016059 (JJW).
Author information
Authors and Affiliations
Contributions
N.K., G.D.R., and J.M.C. wrote the main manuscript text and H.T., J.D.C., and J.J.W. prepared a figure and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
All reported data from human studies by the authors have been compliant with all applicable ethical standards, including the Helsinki Declaration and its amendments, institutional/national research committee standards and guidelines. All mouse studies have been conducted according to approved IACUC protocols.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tenshin, H., Delgado-Calle, J., Windle, J.J. et al. Osteocytes and Paget’s Disease of Bone. Curr Osteoporos Rep 22, 266–272 (2024). https://doi.org/10.1007/s11914-024-00863-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-024-00863-5