Abstract
Purpose of Review
The Runx family genes (Runx1, Runx2, Runx3, and Cbfb) are important transcriptional regulators in the development of various tissues. We herein highlight the roles of the Runx family genes in morphogenesis in the craniofacial regions and in the pathogenesis of congenital morphological problems in these regions.
Recent Findings
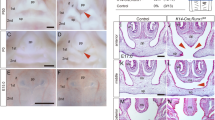
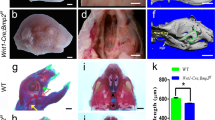
A recent analysis using conditional Runx mutant animals and a human genetic study identified the novel roles of Runx genes in the development of the tooth, salivary glands, and the palate. In an animal study, Runx1/Cbfb signaling was found to regulate the Lgr5 expression and maintain the stem cells in the dental epithelium in the growing incisors. Aberrant Runx1/Cbfb signaling induced male-specific involution of the convoluted granular cell differentiation of the submandibular gland. In palatogenesis, Runx1/Cbfb signaling regulated the Tgfb3 expression in the fusing palatal epithelium through Stat3 activation.
Summary
The combination of a human genetic study and a phenotype analysis of mutant animals revealed the various roles of Runx genes in the development of the tooth, palate, and salivary glands. Runx genes have functional redundancy in various tissues, which still hinder the roles of Runx genes in morphogenesis. Future studies may reveal the novel roles of Runx signaling.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64.
Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–9.
Dinçsoy Bir F, Dinçkan N, Güven Y, Baş F, Altunoğlu U, Kuvvetli SS, et al. Cleidocranial dysplasia: clinical, endocrinologic and molecular findings in 15 patients from 11 families. Eur J Med Genet. 2017;60:163–8. Osteoporosis is common in CCD patients.
Mundlos S. Cleidocranial dysplasia: clinical and molecular genetics. J Med Genet. 1999;36:177–82.
D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, Karsenty G, et al. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–20.
Aberg T, Wang XP, Kim JH, Yamashiro T, Bei M, Rice R, et al. Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol. 2004;270:76–93.
Wheeler JC, Shigesada K, Gergen JP, Ito Y. Mechanisms of transcriptional regulation by Runt domain proteins. Semin Cell Dev Biol. 2000;11:369–75.
Bauer O, Sharir A, Kimura A, Hantisteanu S, Takeda S, Groner Y. Loss of osteoblast Runx3 produces severe congenital osteopenia. Mol Cell Biol. 2015;35:1097–109.
Yamashiro T, Wang XP, Li Z, Oya S, Aberg T, Fukunaga T, et al. Possible roles of Runx1 and Sox9 in incipient intramembranous ossification. J Bone Miner Res. 2004;19:1671–7.
Kimura A, Inose H, Yano F, Fujita K, Ikeda T, Sato S, et al. Runx1 and Runx2 cooperate during sternal morphogenesis. Development. 2010;137:1159–67.
Yoshida CA, Furuichi T, Fujita T, Fukuyama R, Kanatani N, Kobayashi S, et al. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat Genet. 2002;32:633–8.
Ito Y. Molecular basis of tissue-specific gene expression mediated by the runt domain transcription factor PEBP2/CBF. Genes Cells. 1999;4:685–96.
Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–30.
Wang XP, Aberg T, James MJ, Levanon D, Groner Y, Thesleff I. Runx2 (Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and prevents the budding of putative successional teeth. J Dent Res. 2005;84:138–43.
Jensen BL, Kreiborg S. Development of the dentition in cleidocranial dysplasia. J Oral Pathol Med. 1990;19:89–93.
Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103:18627–32.
Wang XP, O’Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, et al. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136:1939–49.
Järvinen E, Shimomura-Kuroki J, Balic A, Jussila M, Thesleff I. Mesenchymal Wnt/β-catenin signaling limits tooth number. Development. 2018;145 Activated canonical Wnt signaling disturbs the sequential tooth formation.
Golan I, Baumert U, Hrala BP, Müssig D. Dentomaxillofacial variability of cleidocranial dysplasia: clinicoradiological presentation and systematic review. Dentomaxillofac Radiol. 2003;32:347–54.
Bronckers AL, Engelse MA, Cavender A, Gaikwad J, D’Souza RN. Cell-specific patterns of Cbfa1 mRNA and protein expression in postnatal murine dental tissues. Mech Dev. 2001;101:255–8.
Chu Q, Gao Y, Gao X, Dong Z, Song W, Xu Z, et al. Ablation of Runx2 in Ameloblasts Suppresses Enamel Maturation in Tooth Development. Sci Rep. 2018;8:9594 Runx2 is essential in enamel maturation.
Yamashiro T, Aberg T, Levanon D, Groner Y, Thesleff I. Expression of Runx1, -2 and -3 during tooth, palate and craniofacial bone development. Mech Dev. 2002;119(Suppl 1):S107–10.
Braddock SR, South ST, Schiffman JD, Longhurst M, Rowe LR, Carey JC. Braddock-Carey syndrome: a 21q22 contiguous gene syndrome encompassing RUNX1. Am J Med Genet A. 2016;170:2580–6.
Sarper SE, Inubushi T, Kurosaka H, Ono Minagi H, Kuremoto KI, Sakai T, et al. Runx1-Stat3 signaling regulates the epithelial stem cells in continuously growing incisors. Sci Rep. 2018;8:10906 Runx1 is involved in the maintenance of the stem cells through regulation of Lgr5 in the cervical loop of the growing incisors.
Kurosaka H, Islam MN, Kuremoto K, Hayano S, Nakamura M, Kawanabe N, et al. Core binding factor beta functions in the maintenance of stem cells and orchestrates continuous proliferation and differentiation in mouse incisors. Stem Cells. 2011;29:1792–803.
Osorio KM, Lee SE, McDermitt DJ, Waghmare SK, Zhang YV, Woo HN, et al. Runx1 modulates developmental, but not injury-driven, hair follicle stem cell activation. Development. 2008;135:1059–68.
Suomalainen M, Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn. 2010;239:364–72.
Minetti CA, Valle LB, Oliveira-Filho RM, Fava-De-Moraes F. Differential actions of testosterone and its metabolites on mice submandibular gland. J Biol Buccale. 1985;13:205–13.
Caramia F. Ultrastructure of mouse submaxillary gland. II. Effect of castration in the male. J Ultrastruct Res. 1966;16:524–36.
Ono Minagi H, Sarper SE, Kurosaka H, Kuremoto KI, Taniuchi I, Sakai T, et al. Runx1 mediates the development of the granular convoluted tubules in the submandibular glands. PLoS One. 2017;12:e0184395 Runx1 regulates the development of the granular convoluted tubules in the submandibular glands.
Islam MN, Itoh S, Yanagita T, Sumiyoshi K, Hayano S, Kuremoto K, et al. Runx/Cbfb signaling regulates postnatal development of granular convoluted tubule in the mouse submandibular gland. Dev Dyn. 2015;244:488–96.
He JF, Ge MH, Zhu X, Chen C, Tan Z, Li YN, et al. Expression of RUNX3 in salivary adenoid cystic carcinoma: implications for tumor progression and prognosis. Cancer Sci. 2008;99:1334–40.
Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–24.
Ge MH, Chen C, Xu JJ, Ling ZQ. Critical regions and spreading of runt-related transcription factor-3 C-phosphate-G (CpG) island methylation in human salivary gland adenoid cystic carcinoma. Hum Pathol. 2011;42:1862–72.
Aberg T, Cavender A, Gaikwad JS, Bronckers AL, Wang X, Waltimo-Sirén J, et al. Phenotypic changes in dentition of Runx2 homozygote-null mutant mice. J Histochem Cytochem. 2004;52:131–9.
Sull JW, Liang KY, Hetmanski JB, Fallin MD, Ingersoll RG, Park J, et al. Differential parental transmission of markers in RUNX2 among cleft case-parent trios from four populations. Genet Epidemiol. 2008;32:505–12.
Wu LZ, Su WQ, Liu YF, Ge X, Zhang Y, Wang XJ. Role of the RUNX2 p.R225Q mutation in cleidocranial dysplasia: a rare presentation and an analysis of the RUNX2 protein structure. Genet Mol Res. 2014;13:1187–94.
Iyyanar PPR, Nazarali AJ. Inhibits bone morphogenetic protein signaling during osteogenic differentiation of the palatal mesenchyme. Front Physiol. 2017;8:929
Sarper SE, Kurosaka H, Inubushi T, Ono Minagi H, Kuremoto KI, Sakai T, et al. Runx1-Stat3-Tgfb3 signaling network regulating the anterior palatal development. Sci Rep. 2018;8:11208. Runx1 regulates the epithelial fusion at the boundary between the primary and the secondary palate.
Braddock SR, Carey JC. A new syndrome: congenital thrombocytopenia, Robin sequence, agenesis of the corpus callosum, distinctive facies and developmental delay. Clin Dysmorphol. 1994;3:75–81.
Hilliard SA, Yu L, Gu S, Zhang Z, Chen YP. Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J Anat. 2005;207:655–67.
Taya Y, O’Kane S, Ferguson MW. Pathogenesis of cleft palate in TGF-beta3 knockout mice. Development. 1999;126:3869–79.
Lane J, Yumoto K, Azhar M, Ninomiya-Tsuji J, Inagaki M, Hu Y, et al. Tak1, Smad4 and Trim33 redundantly mediate TGF-β3 signaling during palate development. Dev Biol. 2015;398:231–41.
Sarper SE, Inubushi T, Kurosaka H, Ono Minagi H, Murata Y, Kuremoto KI, et al. Anterior cleft palate due to Cbfb deficiency and its rescue by folic acid. Dis Model Mech. 2019;12. Cbfb deficiency results in anterior cleft palate and folic acid rescues the mutant phenotype by activation of Stat3 phosphorylation.
Charoenchaikorn K, Yokomizo T, Rice DP, Honjo T, Matsuzaki K, Shintaku Y, et al. Runx1 is involved in the fusion of the primary and the secondary palatal shelves. Dev Biol. 2009;326:392–402.
Khan A, Hyde RK, Dutra A, Mohide P, Liu P. Core binding factor beta (CBFB) haploinsufficiency due to an interstitial deletion at 16q21q22 resulting in delayed cranial ossification, cleft palate, congenital heart anomalies, and feeding difficulties but favorable outcome. Am J Med Genet A. 2006;140:2349–54.
Tsoutsou E, Tzetis M, Giannikou K, Syrmou A, Oikonomakis V, Kosma K, et al. Array-CGH revealed one of the smallest 16q21q22.1 microdeletions in a female patient with psychomotor retardation. Eur J Paediatr Neurol. 2013;17:316–20.
Reich NC. STATs get their move on. JAKSTAT. 2013;2:e27080.
Stark GR, Darnell JE. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–14.
Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702.
Haley KJ, Lasky-Su J, Manoli SE, Smith LA, Shahsafaei A, Weiss ST, et al. RUNX transcription factors: association with pediatric asthma and modulated by maternal smoking. Am J Phys Lung Cell Mol Phys. 2011;301:L693–701.
Wu T, Fallin MD, Shi M, Ruczinski I, Liang KY, Hetmanski JB, et al. Evidence of gene-environment interaction for the RUNX2 gene and environmental tobacco smoke in controlling the risk of cleft lip with/without cleft palate. Birth Defects Res A Clin Mol Teratol. 2012;94:76–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Takashi Yamashiro, Hiroshi Kurosaka, and Toshihiro Inubush declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Craniofacial Skeleton
Rights and permissions
About this article
Cite this article
Yamashiro, T., Kurosaka, H. & Inubush, T. The Association Between Runx Signaling and Craniofacial Development and Disease. Curr Osteoporos Rep 20, 120–126 (2022). https://doi.org/10.1007/s11914-021-00692-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00692-w