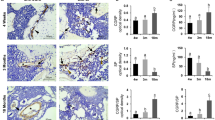
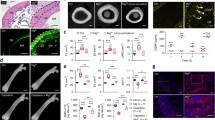
Abstract
Purpose of Review
The goals of this review are two folds: (1) to describe the recent understandings on the roles of calcitonin gene-related peptide-α (CGRP) in bone homeostasis and the underlying mechanisms of related neuronal regulation and (2) to propose innovative CGRP-modulated approaches for enhancing bone regeneration in challenging bone disorders.
Recent Findings
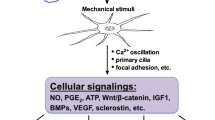
CGRP is predominantly produced by the densely distributed sensory neuronal fibers in bone, declining with age. Under mechanical and biochemical stimulations, CGRP releases and exerts either physiological or pathophysiological roles. CGRP at physiological level orchestrates the communications of bone cells with cells of other lineages, affecting not only osteogenesis, osteoclastogenesis, and adipogenesis but also angiogenesis, demonstrating with pronounced anabolic effect, thus is essential for maintaining bone homeostasis, with tuned nerve-vessel-bone network. In addition, its effects on immunity and cell recruitment are also crucial for bone fracture healing. Binding to the G protein-coupled receptor composited by calcitonin receptor-like receptor (CRLR) and receptor activity modifying protein 1 (RAMP1) on cellular surface, CGRP triggers various intracellular signaling cascades involving cyclic adenosine monophosphate (cAMP) and cAMP response element-binding protein (CREB). Peaking at early stage post-fracture, CGRP promotes bone formation, displaying with larger callus. Then CGRP gradually decreases over time, allowing normal or physiological bone remodeling. By elevating CGRP at early stage, low-intensity pulsed ultrasound (LIPUS), electrical stimulation, and magnesium-based bio-mineral products may promisingly accelerate bone regeneration experimentally in medical conditions like osteoporosis, osteoporotic fracture, and spine fusion. Excess CGRP expression is commonly observed in pathological conditions including cancer metastatic lesions in bone and fracture delayed- or non-healing, resulting in persistent chronic pain. To date, these discoveries have largely been limited to animal models. Clinical applications are highly desirable.
Summary
Compelling evidence show the anabolic effects of CGRP on bone in animals. However, further validation on the role of CGRP and the underlying mechanisms in human skeletons is required. It remains unclear if it is type H vessel connecting neuronal CGRP to osteogenesis, and if there is only specific rather than all osteoprogenitors responsible to CGRP. Clear priority should be put to eliminate these knowledge gaps by integrating with high-resolution 3D imaging of transparent bulk bone and single-cell RNA-sequencing. Last but not the least, given that small molecule antagonists such as BIBN4096BS can block the beneficial effects of CGRP on bone, concerns on the potential side effects of humanized CGRP-neutralizing antibodies when systemically administrated to treat migraine in clinics are arising.






Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D, Chawla A, et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today. 2018;21:362–76.
• Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8 Type H vessels is crucial for bone regeneration.
• Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–80 Endothelial Notch signaling links angiogenesis with osteogenesis.
Maeda Y, Miwa Y, Sato I. Distribution of the neuropeptide calcitonin gene-related peptide-alpha of tooth germ during formation of the mouse mandible. Ann Anat. 2019;221:38–47.
Lerner UH. Deletions of genes encoding calcitonin/alpha-CGRP, amylin and calcitonin receptor have given new and unexpected insights into the function of calcitonin receptors and calcitonin receptor-like receptors in bone. J Musculoskelet Neuronal Interact. 2006;6:87–95.
Chen B, Pei GX, Jin D, Wei KH, Qin Y, Liu QS. Distribution and property of nerve fibers in human long bone tissue. Chin J Traumatol. 2007;10:3–9.
Nencini S, Ivanusic JJ. The Physiology of Bone Pain. How Much Do We Really Know? Front Physiol. 2016;7:157.
•• Brazill JM, Beeve AT, Craft CS, Ivanusic JJ, Scheller EL. Nerves in bone: evolving concepts in pain and anabolism. J Bone Miner Res. 2019;34:1393–406 This review summarizes the physiologcial and pathological roles of both sensory and sympathetic nervous systems on bone.
Li J, Kreicbergs A, Bergstrom J, Stark A, Ahmed M. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: a study in rat angulated tibia. J Orthop Res. 2007;25:1204–12.
Lau YC, Qian X, Po KT, Li LM, Guo X. Electrical stimulation at the dorsal root ganglion preserves trabecular bone mass and microarchitecture of the tibia in hindlimb-unloaded rats. Osteoporos Int. 2015;26:481–8.
Lau YC, Lai YM, Po KT, Qian X, Hao HW, Zhao HC, et al. Dorsal root ganglion electrical stimulation promoted intertransverse process spinal fusion without decortications and bone grafting: a proof-of-concept study. Spine J. 2014;14:2472–8.
Yuen-Chi Lau R, Qian X, Po KT, Li LM, Guo X. Response of rat tibia to prolonged unloading under the influence of electrical stimulation at the dorsal root ganglion. Neuromodulation. 2017;20:284–9.
Naot D, Musson DS, Cornish J. The activity of peptides of the calcitonin family in bone. Physiol Rev. 2019;99:781–805.
Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–142.
Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–35.
Hoppener JW, Steenbergh PH, Zandberg J, Geurts van Kessel AH, Baylin SB, Nelkin BD, et al. The second human calcitonin/CGRP gene is located on chromosome 11. Hum Genet. 1985;70:259–63.
Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–7.
Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195–205.
Hu R, Li YJ, Li XH. An overview of non-neural sources of calcitonin gene-related peptide. Curr Med Chem. 2016;23:763–73.
Fang L, Chen MF, Xiao ZL, Liu Y, Yu GL, Chen XB, et al. Calcitonin gene-related peptide released from endothelial progenitor cells inhibits the proliferation of rat vascular smooth muscle cells induced by angiotensin II. Mol Cell Biochem. 2011;355:99–108.
Ma W, Dumont Y, Vercauteren F, Quirion R. Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line. Immunology. 2010;130:399–409.
Schinke T, Liese S, Priemel M, Haberland M, Schilling AF, Catala-Lehnen P, et al. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res. 2004;19:2049–56.
Ballica R, Valentijn K, Khachatryan A, Guerder S, Kapadia S, Gundberg C, et al. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. J Bone Miner Res. 1999;14:1067–74.
Liu X, Liu H, Xiong Y, Yang L, Wang C, Zhang R, et al. Postmenopausal osteoporosis is associated with the regulation of SP, CGRP, VIP, and NPY. Biomed Pharmacother. 2018;104:742–50.
Valentini A, Petraglia F, De Vita D, Nappi C, Margutti A, Degli Uberti EC, et al. Changes of plasma calcitonin gene-related peptide levels in postmenopausal women. Am J Obstet Gynecol. 1996;175:638–42.
Gao F, Lv TR, Zhou JC, Qin XD. Effects of obesity on the healing of bone fracture in mice. J Orthop Surg Res. 2018;13:145.
Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–6.
• Ramasamy SK, Kusumbe AP, Schiller M, Zeuschner D, Bixel MG, Milia C, et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun. 2016;7:13601 This study inspires future investigations on the CGRP-regulated blood flow on bone formation.
Tomlinson RE, Silva MJ. Skeletal blood flow in bone repair and maintenance. Bone Res. 2013;1:311–22.
Hoheisel U, Mense S, Scherotzke R. Calcitonin gene-related peptide-immunoreactivity in functionally identified primary afferent neurones in the rat. Anat Embryol (Berl). 1994;189:41–9.
Lawson SN, Crepps B, Perl ER. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol. 2002;540:989–1002.
Matteoli M, Haimann C, Torri-Tarelli F, Polak JM, Ceccarelli B, De Camilli P. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc Natl Acad Sci U S A. 1988;85:7366–70.
Peroni RN, Abramoff T, Neuman I, Podesta EJ, Adler-Graschinsky E. Phytoestrogens enhance the vascular actions of the endocannabinoid anandamide in mesenteric beds of female rats. Int J Hypertens. 2012;2012:647856.
Moad HE, Pioszak AA. Selective CGRP and adrenomedullin peptide binding by tethered RAMP-calcitonin receptor-like receptor extracellular domain fusion proteins. Protein Sci. 2013;22:1775–85.
Villa I, Mrak E, Rubinacci A, Ravasi F, Guidobono F. CGRP inhibits osteoprotegerin production in human osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Phys Cell Phys. 2006;291:C529–37.
Uzan B, de Vernejoul MC, Cressent M. RAMPs and CRLR expressions in osteoblastic cells after dexamethasone treatment. Biochem Biophys Res Commun. 2004;321:802–8.
•• Zhang Y, Xu J, Ruan YC, Yu MK, O'Laughlin M, Wise H, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22:1160–9 This study thoroughly explains how magnesium promotes the release of CGRP from sensory neurons and how CGRP promotes osteogenic differentiation of periosteum-derived stem cells.
Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem. 2000;275:31438–43.
Chen H, Hu B, Lv X, Zhu S, Zhen G, Wan M, et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat Commun. 2019;10:181.
Irie K, Hara-Irie F, Ozawa H, Yajima T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech. 2002;58:85–90.
Hukkanen M, Konttinen YT, Rees RG, Gibson SJ, Santavirta S, Polak JM. Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J Rheumatol. 1992;19:1252–9.
Martin CD, Jimenez-Andrade JM, Ghilardi JR, Mantyh PW. Organization of a unique net-like meshwork of CGRP+ sensory fibers in the mouse periosteum: implications for the generation and maintenance of bone fracture pain. Neurosci Lett. 2007;427:148–52.
Sample SJ, Hao Z, Wilson AP, Muir P. Role of calcitonin gene-related peptide in bone repair after cyclic fatigue loading. PLoS One. 2011;6:e20386.
Wang XY, Guo X, Qu SX, Weng J, Cheng CY. Temporal and spatial CGRP innervation in recombinant human bone morphogenetic protein induced spinal fusion in rabbits. Spine (Phila Pa 1976). 2009;34:2363–8.
Li J, Ahmad T, Bergstrom J, Samnegard E, Erlandsson-Harris H, Ahmed M, et al. Differential bone turnover in an angulated fracture model in the rat. Calcif Tissue Int. 2004;75:50–9.
Sample SJ, Heaton CM, Behan M, Bleedorn JA, Racette MA, Hao Z, et al. Role of calcitonin gene-related peptide in functional adaptation of the skeleton. PLoS One. 2014;9:e113959.
He H, Chai J, Zhang S, Ding L, Yan P, Du W, et al. CGRP may regulate bone metabolism through stimulating osteoblast differentiation and inhibiting osteoclast formation. Mol Med Rep. 2016;13:3977–84.
Wang L, Shi X, Zhao R, Halloran BP, Clark DJ, Jacobs CR, et al. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone. 2010;46:1369–79.
Zhou R, Yuan Z, Liu J, Liu J. Calcitonin gene-related peptide promotes the expression of osteoblastic genes and activates the WNT signal transduction pathway in bone marrow stromal stem cells. Mol Med Rep. 2016;13:4689–96.
Akopian A, Demulder A, Ouriaghli F, Corazza F, Fondu P, Bergmann P. Effects of CGRP on human osteoclast-like cell formation: a possible connection with the bone loss in neurological disorders? Peptides. 2000;21:559–64.
Takahashi N, Matsuda Y, Sato K, de Jong PR, Bertin S, Tabeta K, et al. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci Rep. 2016;6:29294.
Maruyama K, Takayama Y, Kondo T, Ishibashi KI, Sahoo BR, Kanemaru H, et al. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-Jdp2 Axis. Cell Rep. 2017;19:2730–42.
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–8.
Xu R, Yallowitz A, Qin A, Wu Z, Shin DY, Kim JM, et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24:823–33.
Chen J, Ma G, Liu W, Liu Y, Ding Y. The influence of the sensory neurotransmitter calcitonin gene-related peptide on bone marrow mesenchymal stem cells from ovariectomized rats. J Bone Miner Metab. 2017;35:473–84.
Li J, Wang Y, Li Y, Sun J, Zhao G. The effect of combined regulation of the expression of peroxisome proliferator-activated receptor-gamma and calcitonin gene-related peptide on alcohol-induced adipogenic differentiation of bone marrow mesenchymal stem cells. Mol Cell Biochem. 2014;392:39–48.
Liu T, Kamiyoshi A, Sakurai T, Ichikawa-Shindo Y, Kawate H, Yang L, et al. Endogenous calcitonin gene-related peptide regulates lipid metabolism and energy homeostasis in male mice. Endocrinology. 2017;158:1194–206.
Aubdool AA, Thakore P, Argunhan F, Smillie SJ, Schnelle M, Srivastava S, et al. A novel alpha-calcitonin gene-related peptide analogue protects against end-organ damage in experimental hypertension, cardiac hypertrophy, and heart failure. Circulation. 2017;136:367–83.
Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–34.
Maeda Y, Miwa Y, Sato I. Expression of CGRP, vasculogenesis and osteogenesis associated mRNAs in the developing mouse mandible and tibia. Eur J Histochem. 2017;61:2750.
Toda M, Suzuki T, Hosono K, Kurihara Y, Kurihara H, Hayashi I, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62:352–9.
Matsui S, Tanaka M, Kamiyoshi A, Sakurai T, Ichikawa-Shindo Y, Kawate H, et al. Endogenous calcitonin gene-related peptide deficiency exacerbates postoperative lymphedema by suppressing lymphatic capillary formation and M2 macrophage accumulation. Am J Pathol. 2019;189:2487–502.
Zheng S, Li W, Xu M, Bai X, Zhou Z, Han J, et al. Calcitonin gene-related peptide promotes angiogenesis via AMP-activated protein kinase. Am J Phys Cell Phys. 2010;299:C1485–92.
Mapp PI, McWilliams DF, Turley MJ, Hargin E, Walsh DA. A role for the sensory neuropeptide calcitonin gene-related peptide in endothelial cell proliferation in vivo. Br J Pharmacol. 2012;166:1261–71.
Toda M, Suzuki T, Hosono K, Hayashi I, Hashiba S, Onuma Y, et al. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc Natl Acad Sci U S A. 2008;105:13550–5.
Liu WC, Chen S, Zheng L, Qin L. Angiogenesis assays for the evaluation of angiogenic properties of orthopaedic biomaterials - a general review. Adv Healthc Mater. 2017;6.
Bo Y, Yan L, Gang Z, Tao L, Yinghui T. Effect of calcitonin gene-related peptide on osteoblast differentiation in an osteoblast and endothelial cell co-culture system. Cell Biol Int. 2012;36:909–15.
Ji G, Xu R, Niu Y, Li N, Ivashkiv L, Bostrom MPG, et al. Vascular endothelial growth factor pathway promotes osseointegration and CD31(hi)EMCN(hi) endothelium expansion in a mouse tibial implant model: an animal study. Bone Joint J. 2019;101-B:108–14.
Ohno T, Hattori Y, Komine R, Ae T, Mizuguchi S, Arai K, et al. Roles of calcitonin gene-related peptide in maintenance of gastric mucosal integrity and in enhancement of ulcer healing and angiogenesis. Gastroenterology. 2008;134:215–25.
Niedermair T, Straub RH, Brochhausen C, Grassel S. Impact of the sensory and sympathetic nervous system on fracture healing in ovariectomized mice. Int J Mol Sci. 2020;21.
Loi F, Cordova LA, Zhang R, Pajarinen J, Lin TH, Goodman SB, et al. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7:15.
Gibon E, Lu LY, Nathan K, Goodman SB. Inflammation, ageing, and bone regeneration. J Orthop Translat. 2017;10:28–35.
Foster CA, Mandak B, Kromer E, Rot A. Calcitonin gene-related peptide is chemotactic for human T lymphocytes. Ann N Y Acad Sci. 1992;657:397–404.
Talme T, Liu Z, Sundqvist KG. The neuropeptide calcitonin gene-related peptide (CGRP) stimulates T cell migration into collagen matrices. J Neuroimmunol. 2008;196:60–6.
Yule KA, White SR. Migration of 3T3 and lung fibroblasts in response to calcitonin gene-related peptide and bombesin. Exp Lung Res. 1999;25:261–73.
Zhang Y, Yang J, Zhang P, Liu T, Xu J, Fan Z, et al. Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Sci Rep. 2016;6:27724.
Wang B, Lin J, Zhang Q, Zhang X, Yu H, Gong P, et al. alphaCGRP affects BMSCs' migration and osteogenesis via the hippo-YAP pathway. Cell Transplant. 2019;28:1420–31.
Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol. 2008;8:460–71.
Walsh DA, Mapp PI, Kelly S. Calcitonin gene-related peptide in the joint: contributions to pain and inflammation. Br J Clin Pharmacol. 2015;80:965–78.
Miranda LP, Holder JR, Shi L, Bennett B, Aral J, Gegg CV, et al. Identification of potent, selective, and metabolically stable peptide antagonists to the calcitonin gene-related peptide (CGRP) receptor. J Med Chem. 2008;51:7889–97.
Khan Y, Laurencin CT. Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am. 2008;90(Suppl 1):138–44.
Zhang N, Chow SK, Leung KS, Cheung WH. Ultrasound as a stimulus for musculoskeletal disorders. J Orthop Translat. 2017;9:52–9.
Lam WL, Guo X, Leung KS, Kwong KS. The role of the sensory nerve response in ultrasound accelerated fracture repair. J Bone Joint Surg (Br). 2012;94:1433–8.
Wang XY, Guo X, Cheng JC, Mi YL, Lai PY. Involvement of calcitonin gene-related peptide innervation in the promoting effect of low-intensity pulsed ultrasound on spinal fusion without decortication. Spine (Phila Pa 1976). 2010;35:E1539–45.
Ding L, Song T, Yi C, Huang Y, Yu W, Ling L, et al. Transcutaneous electrical nerve stimulation (TENS) improves the diabetic cytopathy (DCP) via up-regulation of CGRP and cAMP. PLoS One. 2013;8:e57477.
Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM, et al. Neuromodulation. 2013;16:67–71 discussion −2.
Seagrove LC, Suzuki R, Dickenson AH. Electrophysiological characterisations of rat lamina I dorsal horn neurones and the involvement of excitatory amino acid receptors. Pain. 2004;108:76–87.
Ariza AC, Bobadilla N, Diaz L, Avila E, Larrea F, Halhali A. Placental gene expression of calcitonin gene-related peptide and nitric oxide synthases in preeclampsia: effects of magnesium sulfate. Magnes Res. 2009;22:44–9.
de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46.
Zhao D, Witte F, Lu F, Wang J, Li J, Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2016;112:287–302.
Lin X, Ge J, Wei D, Liu C, Tan L, Yang H, et al. Surface degradation-enabled osseointegrative, angiogenic and antiinfective properties of magnesium-modified acrylic bone cement. J Orthop Translat. 2019;17:121–32.
Song B, Li W, Chen Z, Fu G, Li C, Liu W, et al. Biomechanical comparison of pure magnesium interference screw and polylactic acid polymer interference screw in anterior cruciate ligament reconstruction—a cadaveric experimental study. J Orthop Translat. 2017;8:32–9.
Cheng P, Han P, Zhao C, Zhang S, Wu H, Ni J, et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26.
Wang J, Xu J, Song B, Chow DH, Shu-Hang Yung P, Qin L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017;63:393–410.
Wang J, Xu J, Fu W, Cheng W, Chan K, Yung PS, et al. Biodegradable magnesium screws accelerate fibrous tissue mineralization at the tendon-bone insertion in anterior cruciate ligament reconstruction model of rabbit. Sci Rep. 2017;7:40369.
Chen XD. Magnesium-based implants: beyond fixators. J Orthop Transl. 2017;10:1–4.
Zheng LZ, Wang JL, Xu JK, Zhang XT, Liu BY, Huang L, et al. Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model. Biomaterials. 2020;238:119828.
Zhao D, Huang S, Lu F, Wang B, Yang L, Qin L, et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016;81:84–92.
Zhu S, Zhu J, Zhen G, Hu Y, An S, Li Y, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. 2019;129:1076–93.
Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–12.
Bullock CM, Kelly S. Calcitonin gene-related peptide receptor antagonists: beyond migraine pain—a possible analgesic strategy for osteoarthritis? Curr Pain Headache Rep. 2013;17:375.
Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthr Cartil. 2014;22:578–85.
Nakasa T, Ishikawa M, Takada T, Miyaki S, Ochi M. Attenuation of cartilage degeneration by calcitonin gene-related paptide receptor antagonist via inhibition of subchondral bone sclerosis in osteoarthritis mice. J Orthop Res. 2016;34:1177–84.
•• Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain. 2014;155:2323–36 Delayed or non-healed fracture callus also present with aberrant distribution of sensory nerve fibers.
Jimenez-Andrade JM, Bloom AP, Mantyh WG, Koewler NJ, Freeman KT, Delong D, et al. Capsaicin-sensitive sensory nerve fibers contribute to the generation and maintenance of skeletal fracture pain. Neuroscience. 2009;162:1244–54.
Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–98.
Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30:14649–56.
Bloom AP, Jimenez-Andrade JM, Taylor RN, Castaneda-Corral G, Kaczmarska MJ, Freeman KT, et al. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain. 2011;12:698–711.
Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809.
Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol. 2014;32:1647–54.
Zhou XY, Xu XM, Wu SY, Wang F, Yang YL, Li M, et al. Spatiotemporal changes of calcitonin gene-related peptide innervation in spinal fusion. Biomed Res Int. 2016;2016:5872860.
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23.
Orita S, Ohtori S, Koshi T, Yamashita M, Yamauchi K, Inoue G, et al. The effects of risedronate and exercise on osteoporotic lumbar rat vertebrae and their sensory innervation. Spine (Phila Pa 1976). 2010;35:1974–82.
Yu D, Liu F, Liu M, Zhao X, Wang X, Li Y, et al. The inhibition of subchondral bone lesions significantly reversed the weight-bearing deficit and the overexpression of CGRP in DRG neurons, GFAP and Iba-1 in the spinal dorsal horn in the monosodium iodoacetate induced model of osteoarthritis pain. PLoS One. 2013;8:e77824.
•• Greenbaum A, Chan KY, Dobreva T, Brown D, Balani DH, Boyce R, et al. Bone CLARITY: clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Sci Transl Med. 2017;9. Advanced imaging technology would provide more precise information than ever before.
•• Wang Q, Liu K, Yang L, Wang H, Yang J. BoneClear: whole-tissue immunolabeling of the intact mouse bones for 3D imaging of neural anatomy and pathology. Cell Res. 2019;29:870–2 Advanced imaging technology would provide more precise information than ever before.
Funding
The authors were supported by Hong Kong RGC Theme-based Research Scheme (2017/18, T13-402/17-N), National Natural Science Foundation of China (81802152 and 81702165), Collaborative Research Fund (C4026-17WF), and Health & Medical Research Fund (17180671).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have declared that no competing interest exists.
Human and Animal Rights and Informed Consent
All reported experiments with animal subjects performed by the authors have been previously published and complied with ethical standards including the Helsinki declaration and its amendments, institutional/national research committee standards and guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Bone and Joint Pain
Rights and permissions
About this article
Cite this article
Xu, J., Wang, J., Chen, X. et al. The Effects of Calcitonin Gene-Related Peptide on Bone Homeostasis and Regeneration. Curr Osteoporos Rep 18, 621–632 (2020). https://doi.org/10.1007/s11914-020-00624-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00624-0