Abstract
Purpose of Review
In this review, we present the application of pragmatic clinical trials for evaluating interventions in osteoporosis, and we discuss methodological considerations for designing and conducting a pragmatic clinical trial compared with a classical randomized clinical trial.
Recent Findings
Pragmatic clinical trials are a popular study design testing effectiveness of health interventions and are intended to address the limitations associated with traditional explanatory randomized clinical trials testing efficacy of interventions. To date, only few pragmatic clinical trials have been conducted in osteoporosis.
Summary
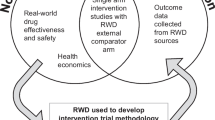
Pragmatic clinical trials are conducted under routine clinical practice setting and are intended to inform policy makers and clinical decisions. Osteoporosis is a chronic disease well-suited to this particular study design given the existence of a clear and specific natural endpoint, namely fracture occurrence, and the availability of several treatments to prevent fractures.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20:637–48. https://doi.org/10.1016/0021-9681(67)90041-0.
Dal-Ré R, Janiaud P, Ioannidis JPA. Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic? BMC Med. 2018;16:49. https://doi.org/10.1186/s12916-018-1038-2.
Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–75. https://doi.org/10.1016/j.jclinepi.2008.12.011.
•• Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. https://doi.org/10.1136/bmj.h2147This manuscript provides the definition of the PRECIS-2 tool. The PRECIS-2 tool is intended to help trialists design their clinical trials.
Black DM, Rosen CJ. Clinical practice. Postmenopausal Osteoporosis. N Engl J Med. 2016;374:254–62. https://doi.org/10.1056/NEJMcp1513724.
Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. https://doi.org/10.1186/s13063-015-1023-4.
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–43. https://doi.org/10.1056/NEJM199511303332201.
Rossini M, Adami G, Adami S, Viapiana O, Gatti D. Safety issues and adverse reactions with osteoporosis management. Expert Opin Drug Saf. 2016;15:321–32. https://doi.org/10.1517/14740338.2016.1136287.
Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230–40. https://doi.org/10.1016/S0140-6736(17)32137-2.
Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. https://doi.org/10.1002/jbmr.5650080915.
Kim SY, Flory J, Relton C. Ethics and practice of trials within cohorts: an emerging pragmatic trial design. Clin Trials. 2018;15:9–16. https://doi.org/10.1177/1740774517746620.
Anderson EE, Newman SB, Matthews AK. Improving informed consent: stakeholder views. AJOB Empir Bioeth. 2017;8:178–88. https://doi.org/10.1080/23294515.2017.1362488.
• Warriner AH, Foster PJ, Mudano A, Wright NC, Melton ME, Sattui SE, et al. A pragmatic randomized trial comparing tablet computer informed consent to traditional paper-based methods for an osteoporosis study. Contemp Clin Trials Commun. 2016;3:32–8. https://doi.org/10.1016/j.conctc.2016.02.003This manuscript presents the results of a PCT comparing eConsent and paper-based consent in osteoporosis.
Junghans C, Jones M. Consent bias in research: how to avoid it. Heart. 2007;93:1024–5. https://doi.org/10.1136/hrt.2007.120113.
Yen P-Y, Sousa KH, Bakken S. Examining construct and predictive validity of the Health-IT Usability Evaluation Scale: confirmatory factor analysis and structural equation modeling results. J Am Med Inform Assoc. 2014;21:e241–8. https://doi.org/10.1136/amiajnl-2013-001811.
Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–47. https://doi.org/10.1093/jnci/93.2.139.
•• Department of Health and Human Services (HHS). Federal policy for the protection of human subjects (final rule). 82 Federal Register 7149, January 19, 2017., (2017).The "Common Rule" governs the ethical conduct of research.
Sugarman J, Califf RM. Ethics and regulatory complexities for pragmatic clinical trials. JAMA. 2014;311:2381–2. https://doi.org/10.1001/jama.2014.4164.
Ellenberg SS. Randomization designs in comparative clinical trials. N Engl J Med. 1984;310:1404–8. https://doi.org/10.1056/NEJM198405243102141.
Fahed R, Finitsis S, Khoury N, Deschaintre Y, Daneault N, Gioia L, et al. A randomized pragmatic care trial on endovascular acute stroke interventions (EASI): criticisms, responses, and ethics of integrating research and clinical care. Trials. 2018;19:508. https://doi.org/10.1186/s13063-018-2870-6.
McRae AD, Taljaard M, Weijer C. Cluster-randomized trials: a closer look. Clin Trials. 2016;13:294–300. https://doi.org/10.1177/1740774516629405.
Kerger BD, Bernal A, Paustenbach DJ, Huntley-Fenner G. Halo and spillover effect illustrations for selected beneficial medical devices and drugs. BMC Public Health. 2016;16:979. https://doi.org/10.1186/s12889-016-3595-7.
Edmonds SW, Wolinsky FD, Christensen AJ, Lu X, Jones MP, Roblin DW, et al. The PAADRN study: a design for a randomized controlled practical clinical trial to improve bone health. Contemp Clin Trials. 2013;34:90–100. https://doi.org/10.1016/j.cct.2012.10.002.
Cram P, Wolinsky FD, Lou Y, Edmonds SW, Hall SF, Roblin DW, et al. Patient-activation and guideline-concordant pharmacological treatment after bone density testing: the PAADRN randomized controlled trial. Osteoporos Int. 2016;27:3513–24. https://doi.org/10.1007/s00198-016-3681-9.
Pablos-Méndez A, Barr RG, Shea S. Run-in periods in randomized trials: implications for the application of results in clinical practice. JAMA. 1998;279:222–5.
• Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19:370–8. https://doi.org/10.1359/JBMR.0301240This manuscript presents a PCT that tested the efficacy of vitamin D and calcium in more than 9000 community-dwelling residents aged more than 66 years.
Robertson L, Black C, Fluck N, Gordon S, Hollick R, Nguyen H, et al. Hip fracture incidence and mortality in chronic kidney disease: the GLOMMS-II record linkage cohort study. BMJ Open. 2018;8:e020312. https://doi.org/10.1136/bmjopen-2017-020312.
Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. National Osteoporosis Guideline Group (NOGG), UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. https://doi.org/10.1007/s11657-017-0324-5.
Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int. 2019;30:1145–56. https://doi.org/10.1007/s00198-019-04906-x.
Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–39. https://doi.org/10.1056/NEJMoa071408.
Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018;6:445–54. https://doi.org/10.1016/S2213-8587(18)30075-5.
Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42:2309–18. https://doi.org/10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K.
•• Colman EG. The Food and Drug Administration’s osteoporosis guidance document: past, present, and future. J Bone Miner Res. 2003;18:1125–8. https://doi.org/10.1359/jbmr.2003.18.6.1125The Food and Drug Administration guidance for the design of clinical trials in osteoporosis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Giovanni Adami, Kenneth G. Saag, and Maria I. Danila declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality of Care in Osteoporosis
Rights and permissions
About this article
Cite this article
Adami, G., Saag, K.G. & Danila, M.I. Pragmatic Clinical Trials in Osteoporosis. Curr Osteoporos Rep 17, 521–526 (2019). https://doi.org/10.1007/s11914-019-00551-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-019-00551-9