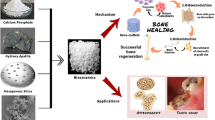
Abstract
Osteoporosis is a degenerative bone disease commonly related to aging. With an increase in life expectancies worldwide, the prevalence of the disease is expected to rise. Current clinical therapeutic treatments are not able to offer long-term solutions to counter the bone mass loss and the increased risk of fractures, which are the primary characteristics of the disease. However, the combination of bioactive nanomaterials within a biomaterial scaffold shows promise for the development of a localized, long-term treatment for those affected by osteoporosis. This review summarizes the unique characteristics of engineered nanoparticles that render them applicable for bone regeneration and recaps the current body of knowledge on nanomaterials with potential for osteoporosis treatment and bone regeneration. Specifically, we highlight new developments that are shaping this emerging field and evaluate applications of recently developed nanomaterials for osteoporosis treatment. Finally, we will identify promising new research directions in nanotechnology for bone regeneration.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Felsenberg D, Silman A, Lunt M, Armbrecht G, Ismail A, Finn J, et al. Incidence of vertebral fracture in Europe: results from the European prospective osteoporosis study (EPOS). J Bone Miner Res. 2002;17:716–24.
Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33.
Mackey PA, Whitaker MD. Osteoporosis: a therapeutic update. J Nurs Pract. 2015;11:1011–7.
Ehrlich P, Lanyon L. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700.
Parfitt AM. Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med. 1987;82:68–72.
Riggs BL, Khosla S, Melton LJ. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–73.
Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–5.
Francis R. The effects of testosterone on osteoporosis in men. Clin Endocrinol. 1999;50:411–4.
Zallone A. Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann N Y Acad Sci. 2006;1068:173–9.
Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300.
Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122:S14–21.
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437–44.
Lozano-Calderon SA, Colman MW, Raskin KA, Hornicek FJ, Gebhardt M. Use of bisphosphonates in orthopedic surgery: pearls and pitfalls. Orthop Clin N Am. 2014;45:403–16.
Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67:994–1001.
Alghamdi HS, Bosco R, Both SK, Iafisco M, Leeuwenburgh SC, Jansen JA, et al. Synergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic rats. Biomaterials. 2014;35:5482–90. Nanoparticles are utilized on the surface of an implant to concurrently promote osteoblast activity while also decreasing osteoclast activity.
Bosco R, Iafisco M, Tampieri A, Jansen JA, Leeuwenburgh SC, van den Beucken JJ. Hydroxyapatite nanocrystals functionalized with alendronate as bioactive components for bone implant coatings to decrease osteoclastic activity. Appl Surf Sci. 2015;328:516–24. This study highlights the use of hydroxyapatite nanoparticles as not only an osteoconductive material but also as a delivery vehicle for a drug to decrease osteoclast activity.
Diab DL, Watts NB. Bisphosphonates in the treatment of osteoporosis. Endocrinol Metab Clin N Am. 2012;41:487–506.
Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14.
Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35:1794–800.
Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91.
Lad SP, Nathan JK, Boakye M. Trends in the use of bone morphogenetic protein as a substitute to autologous iliac crest bone grafting for spinal fusion procedures in the United States. Spine. 2011;36:E274–81.
Ehnert S, Jian Z, Pscherer S, Freude T, Dooley S, Kolk A, et al. Transforming growth factor b1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Med. 2012;10:101–11.
Karunaratne DN. Nanotechnology in medicine. Journal of the National Science Foundation of Sri Lanka. 2010;35:149–52.
Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111:441–53.
Rawat M, Singh D, Saraf S, Saraf S. Nanocarriers: promising vehicle for bioactive drugs. Biol Pharm Bull. 2006;29:1790–8.
Carrow JK, Gaharwar AK. Bioinspired polymeric nanocomposites for regenerative medicine. Macromol Chem Phys. 2015;216:248–64.
Kerativitayanan P, Carrow JK, Gaharwar AK. Nanomaterials for engineering stem cell responses. Adv Healthc Mater. 2015;4:1600–27.
Tran N, Webster TJ. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater. 2011;7:1298–306.
Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4:66–80.
Webste T. Nanophase ceramics: the future orthopedic and dental implant material. In: Ying J, editor. Advances in chemical engineering, vol. 27. New York: Academic Press; 2001. p. 125–66.
Fricain JC, Schlaubitz S, Le Visage C, Arnault I, Derkaoui SM, Siadous R, et al. A nano-hydroxyapatite–pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials. 2013;34:2947–59. When tested in several in vivo models, the composite scaffold induces high mineralization as well as maintains incorporated growth factors.
Xu A, Liu X, Gao X, Deng F, Deng Y, Wei S. Enhancement of osteogenesis on micro/nano-topographical carbon fiber-reinforced polyetheretherketone–nanohydroxyapatite biocomposite. Mater Sci Eng C. 2015;48:592–8. By incorporating nanohydroxyapatite into the composite, stem cell osteo-differentiation, mineralization, and interaction with the composite are increased.
Chimene D, Alge DL, Gaharwar AK. Two‐dimensional nanomaterials for biomedical applications: emerging trends and future prospects. Adv Mater. 2015;27:7261–84.
Gaharwar AK, Dammu SA, Canter JM, Wu C-J, Schmidt G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly (ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules. 2011;12:1641–50.
Hu Y, Cai K, Luo Z, Jandt KD. Layer‐by‐layer assembly of β‐estradiol loaded mesoporous silica nanoparticles on titanium substrates and its implication for bone homeostasis. Adv Mater. 2010;22:4146–50.
Sowjanya J, Singh J, Mohita T, Sarvanan S, Moorthi A, Srinivasan N, et al. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf B: Biointerfaces. 2013;109:294–300.
Tripathi A, Saravanan S, Pattnaik S, Moorthi A, Partridge NC, Selvamurugan N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper–zinc for bone tissue engineering. Int J Biol Macromol. 2012;50:294–9.
Kang G, Wang Y, Liu J, Wu J, Zhao M, Li G, et al. Development of three-component conjugates: to get nano-globes with porous surfaces, high in vivo anti-osteoporosis activity and minimal side effects. J Mater Chem. 2012;22:21740–8.
Cao L, Wang J, Hou J, Xing W, Liu C. Vascularization and bone regeneration in a critical sized defect using 2-N, 6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials. 2014;35:684–98. Demonstrates ability of nanoparticle system to deliver BMP-2 for short and long-term treatment to improve bone regeneration as well as promote vascularization.
Ignjatović N, Ajduković Z, Savić V, Najman S, Mihailović D, Vasiljević P, et al. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J Mater Sci Mater Med. 2013;24:343–54.
Weitzmann MN, Ha S-W, Vikulina T, Roser-Page S, Lee J-K, Beck GR. Bioactive silica nanoparticles reverse age-associated bone loss in mice. Nanomed Nanotechnol Biol Med. 2015;11:959–67. Successful in vivo mice studies show potential for administration of silica nanoparticles to counteract age-related bone loss.
Kim T-H, Singh RK, Kang MS, Kim J-H, Kim H-W. Inhibition of osteoclastogenesis through siRNA delivery with tunable mesoporous bioactive nanocarriers. Acta Biomater. 2015. siRNA is incorporated into bioglass nanospheres, which are biocompatible and biodegradable, and successfully delivered to inhibit osteoclast activity.
Saravanan S, Sameera D, Moorthi A, Selvamurugan N. Chitosan scaffolds containing chicken feather keratin nanoparticles for bone tissue engineering. Int J Biol Macromol. 2013;62:481–6.
Gaharwar AK, Mihaila SM, Swami A, Patel A, Sant S, Reis RL, et al. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv Mater. 2013;25:3329–36.
Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, et al. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano. 2015;9:3109–18. Incorporation of two-dimensional silicate nanoparticles not only increases the structural properties of the scaffold but provides a growth-factor free approach to stimulating osteogenic differentiation.
Liu Y, Lu Y, Tian X, Cui G, Zhao Y, Yang Q, et al. Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials. 2009;30:6276–85.
El-Fiqi A, Kim H-W. Mesoporous bioactive nanocarriers in electrospun biopolymer fibrous scaffolds designed for sequential drug delivery. RSC Adv. 2014;4:4444–52.
Kang MS, Kim J-H, Singh RK, Jang J-H, Kim H-W. Therapeutic-designed electrospun bone scaffolds: Mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015;16:103–16. A nanocomposite system is designed to deliver two different growth factors at two different rates; the nanocarriers allowed for sustained release of the later acting growth factor.
Crowder SW, Prasai D, Rath R, Balikov DA, Bae H, Bolotin KI, et al. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2013;5:4171–6. Graphene is novel two-dimensional material and its incorporation into a three-dimensional matrix not only improves the scaffolds mechanical properties but also enhances stem cell osteogenic differentiation.
Thakur T, Xavier JR, Cross L, Jaiswal MK, Mondragon E, Kaunas R, et al. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J Biomed Mater Res A. 2016;104(4):879–888.
Kerativitayanan P, Gaharwar AK. Elastomeric and mechanically stiff nanocomposites from poly (glycerol sebacate) and bioactive nanosilicates. Acta Biomater. 2015;26:34–44.
Gaharwar AK, Mukundan S, Karaca E, Dolatshahi-Pirouz A, Patel A, Rangarajan K, et al. Nanoclay-enriched poly (ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2014;20(15–16):2088–2101.
Saifullah B, Arulselvan P, El Zowalaty ME, Fakurazi S, Webster TJ, Geilich BM, et al. Development of a biocompatible nanodelivery system for tuberculosis drugs based on isoniazid-Mg/Al layered double hydroxide. Int J Nanomedicine. 2014;9:4749.
Tran PA, Sarin L, Hurt RH, Webster TJ. Opportunities for nanotechnology-enabled bioactive bone implants. J Mater Chem. 2009;19:2653–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mikayla Barry, Hannah Pearce, Lauren Cross, Marco Tatullo, and Akhilesh K. Gaharwar declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Regenerative Biology and Medicine in Osteoporosis
Mikayla Barry and Hannah Pearce contributed equally to this work.
Rights and permissions
About this article
Cite this article
Barry, M., Pearce, H., Cross, L. et al. Advances in Nanotechnology for the Treatment of Osteoporosis. Curr Osteoporos Rep 14, 87–94 (2016). https://doi.org/10.1007/s11914-016-0306-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-016-0306-3