Abstract
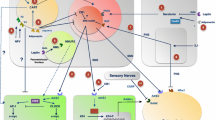
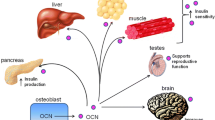
Neural pathways are now a well-appreciated factor in the regulatory milieu controlling the maintenance of bone mass. A number of neural pathways from the brain to bone have been identified. These pathways often involve elements of the energy homeostatic apparatus, indicating links between the regulation of bone metabolism and energy balance. Neuropeptide Y is one such factor that co-regulates these two processes. Initial studies outlined the skeletal actions of NPY from within the brain and the interactions with energy homeostatic processes. However, in recent years, an appreciation for the actions of NPY within bone cells has expanded. Cells of the osteoblastic lineage express both NPY ligand and a cognate receptor NPY, Y1R. Murine studies have demonstrated that both ligand and receptor actively control bone mass and osteoblast activity and interact with mechanical signals to integrate with the local loading environment. Local NPY signalling regulates osteoprogenitor production and differentiation, to cover the entire osteoblastic lineage. In addition, several recent studies have demonstrated extra-skeletal actions of osteoblastic NPY signalling, to regulate energy expenditure and with it adiposity, and in a separate study, to control release of a factor-controlling beta cell mass and insulin production/release and with it glucose tolerance. Thus, osteoblastic neuropeptide production and signalling illustrates the rapidly widening sphere of influence of skeletal tissue, and suggests a far more complex and interconnected physiology then is currently appreciated.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207.
Baraban SC. Neuropeptide Y, and limbic seizures. Rev Neurosci. 1998;9(2):117–28.
Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38(4):189–200. doi:10.1016/j.npep.2004.05.005.
Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8(3):225–35.
Igwe JC, Jiang X, Paic F, Ma L, Adams DJ, Baldock PA, et al. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J Cell Biochem. 2009;108(3):621–30. doi:10.1002/jcb.22294.
Driessler F, Baldock PA. Hypothalamic regulation of bone. J Mol Endocrinol. 2010;45(4):175–81. doi:10.1677/jme-10-0015.
Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, et al. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One. 2009;4(12):e8415. doi:10.1371/journal.pone.0008415.
Baldock PA, Allison SJ, Lundberg P, Lee NJ, Slack K, Lin EJ, et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J Biol Chem. 2007;26:19092–102. United States.
Yang K, Guan H, Arany E, Hill DJ, Cao X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008;22(7):2452–64. doi:10.1096/fj.07-100735.
Singer K, Morris DL, Oatmen KE, Wang T, Del Proposto J, Mergian T, et al. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS One. 2013;8(3):e57929. doi:10.1371/journal.pone.0057929.
Blomqvist AG, Herzog H. Y-receptor subtypes—how many more? Trends Neurosci. 1997;20(7):294–8.
Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11(4):1431–48.
Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13(7):803–11. doi:10.1038/nm1611.
Lee NJ, Doyle KL, Sainsbury A, Enriquez RF, Hort YJ, Riepler SJ, et al. Critical role for Y1 receptors in mesenchymal progenitor cell differentiation and osteoblast activity. J Bone Miner Res. 2010;25(8):1736–47. doi:10.1002/jbmr.61.
Lundberg P, Allison SJ, Lee NJ, Baldock PA, Brouard N, Rost S, et al. Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J Biol Chem. 2007;26:19082–91. United States.
Baldock PA, Sainsbury A, Allison S, Lin EJ, Couzens M, Boey D, et al. Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. J Bone Miner Res. 2005;20(10):1851–7. doi:10.1359/jbmr.050523.
Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, et al. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111(3):443–532.
Allison SJ, Baldock P, Sainsbury A, Enriquez R, Lee NJ, Lin EJ, et al. Conditional deletion of hypothalamic Y2 receptors reverts gonadectomy-induced bone loss in adult mice. J Biol Chem. 2006;281(33):23436–44.
Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, et al. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109(7):915–21. doi:10.1172/jci14588.
Burcelin R, Brunner H, Seydoux J, Thorensa B, Pedrazzini T. Increased insulin concentrations and glucose storage in neuropeptide Y Y1 receptor-deficient mice. Peptides. 2001;22(3):421–7.
Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc Natl Acad Sci U S A. 1998;95(26):15659–64.
Pedrazzini T, Seydoux J, Kunstner P, Aubert JF, Grouzmann E, Beermann F, et al. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4(6):722–6.
Lundberg JM, Fried G, Pernow J, Theodorsson-Norheim E. Co-release of neuropeptide Y and catecholamines upon adrenal activation in the cat. Acta Physiol Scand. 1986;126(2):231–8. doi:10.1111/j.1748-1716.1986.tb07810.x.
Kempna P, Korner M, Waser B, Hofer G, Nuoffer JM, Reubi JC, et al. Neuropeptide Y modulates steroid production of human adrenal H295R cells through Y1 receptors. Mol Cell Endocrinol. 2010;314(1):101–9. doi:10.1016/j.mce.2009.08.010.
Morgan DG, Kulkarni RN, Hurley JD, Wang ZL, Wang RM, Ghatei MA, et al. Inhibition of glucose stimulated insulin secretion by neuropeptide Y is mediated via the Y1 receptor and inhibition of adenylyl cyclase in RIN 5AH rat insulinoma cells. Diabetologia. 1998;41(12):1482–91. doi:10.1007/s001250051095.
Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45(4):682–92. doi:10.1016/j.bone.2009.06.010.
Amano S, Arai M, Goto S, Togari A. Inhibitory effect of NPY on isoprenaline-induced osteoclastogenesis in mouse bone marrow cells. Biochim Biophys Acta. 2007;1770(6):966–73. doi:10.1016/j.bbagen.2007.02.009.
Lee NJ, Nguyen AD, Enriquez RF, Luzuriaga J, Bensellam M, Laybutt R, et al. NPY signalling in early osteoblasts controls glucose homeostasis. Mol Metab. 2015;3:164–74. Germany, this study blocked NPY signalling through Y1R deletion in early osteoblasts and demonstrated a marked alteration in beta cell development and glucose metabolism in adult mice. This effect was absent in a later Y1R knockout, confirming an osteocalcin-independent effect, and one that was magnified in obese animals, highlighting the interrelationship between bone and glucose metabolism, and the potential for therapeutic development of inter-organ endocrine signalling molecules.
Lee NJ, Nguyen AD, Enriquez RF, Doyle KL, Sainsbury A, Baldock PA, et al. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone. 2011;3:461–7. United States: Elsevier Inc.
Sousa DM, Baldock PA, Enriquez RF, Zhang L, Sainsbury A, Lamghari M, et al. Neuropeptide Y Y1 receptor antagonism increases bone mass in mice. Bone. 2012;51(1):8–16. doi:10.1016/j.bone.2012.03.020. First study to indicate the potential therapeutic promise of an orally administered Y1 receptor antagonist.
Sousa DM, McDonald MM, Mikulec K, Peacock L, Herzog H, Lamghari M, et al. Neuropeptide Y modulates fracture healing through Y1 receptor signaling. J Orthop Res. 2013;31(10):1570–8. doi:10.1002/jor.22400.
Teixeira L, Sousa DM, Nunes AF, Sousa MM, Herzog H, Lamghari M. NPY revealed as a critical modulator of osteoblast function in vitro: new insights into the role of Y1 and Y2 receptors. J Cell Biochem. 2009;107(5):908–16. doi:10.1002/jcb.22194.
Matic I, Matthews BG, Kizivat T, Igwe JC, Marijanovic I, Ruohonen ST, et al. Bone-specific overexpression of NPY modulates osteogenesis. J Musculoskelet Neuronal Interact. 2012;12(4):209–18. The first study to specifically alter osteoblastic NPY production and demonstrate a paracrine/autocrine suppression of bone formation and reduction in bone mass. Thereby reinforcing the previous studies showing stimulation of bone formation with deletion of local NPY, Y1R from osteoblasts.
Baldock PA, Lin S, Zhang L, Karl T, Shi Y, Driessler F, et al. Neuropeptide y attenuates stress-induced bone loss through suppression of noradrenaline circuits. J Bone Miner Res. 2014;29(10):2238–49. doi:10.1002/jbmr.2205.
Rush RA, Geffen LB. Dopamine beta-hydroxylase in health and disease. Crit Rev Clin Lab Sci. 1980;12(3):241–77. doi:10.3109/10408368009108731.
Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, et al. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66(7):705–7. doi:10.1016/j.biopsych.2009.04.037.
Heilig M. The NPY, system in stress, anxiety and depression. Neuropeptides. 2004;38(4):213–24. doi:10.1016/j.npep.2004.05.002.
Zhang L, Macia L, Turner N, Enriquez RF, Riepler SJ, Nguyen AD, et al. Peripheral neuropeptide Y Y1 receptors regulate lipid oxidation and fat accretion. Int J Obes. 2010;34(2):357–73. doi:10.1038/ijo.2009.232.
Rodriguez-Carballo E, Gamez B, Mendez-Lucas A, Sanchez-Freutrie M, Zorzano A, Bartrons R, et al. p38alpha function in osteoblasts influences adipose tissue homeostasis. FASEB J. 2015;4:1414–25. United States: FASEB First study to demonstrate a change in osteoblastic NPY production and release was associated with changes in regulation of other systems, in this case energy homeostasis and adiposity. Previous studies had only examined bone changes with local NPY over expression by transgene [33].
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Curr Osteoporos Rep. 2015;13(3):180–5. doi:10.1007/s11914-015-0267-y.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors of this paper declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Skeletal Biology and Regulation
Rights and permissions
About this article
Cite this article
Horsnell, H., Baldock, P.A. Osteoblastic Actions of the Neuropeptide Y System to Regulate Bone and Energy Homeostasis. Curr Osteoporos Rep 14, 26–31 (2016). https://doi.org/10.1007/s11914-016-0300-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-016-0300-9