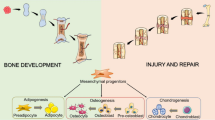
Abstract
Osteoblasts are an important cellular component of the bone microenvironment controlling bone formation and hematopoiesis. Understanding the cellular and molecular mechanisms by which osteoblasts regulate these processes is a rapidly growing area of research given the important implications for bone therapy, regenerative medicine, and hematopoietic stem cell transplantation. Here we summarize our current knowledge regarding the cellular and molecular crosstalk driving bone formation and hematopoiesis and will discuss the implications of a recent finding demonstrating that osteoblasts are a cellular source of erythropoietin .
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201.
Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–44.
Chan CK, Chen CC, Luppen CA, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–4.
Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64.
Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–71.
Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29.
Gerber HP, Vu TH, Ryan AM, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–8.
Maes C, Carmeliet P, Moermans K, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73.
Zelzer E, McLean W, Ng YS, et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–904.
Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8. This paper identifies a specific population of endothelial cells in bone that are responsible for the coupling of angiogenesis and osteogenesis.
Ramasamy SK, Kusumbe AP, Wang L, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–80. This paper demonstrates that Notch signaling in endothelial cells regulates osteoblast differentiation through the production of Noggin.
Schipani E, Wu C, Rankin EB, et al. Regulation of bone marrow angiogenesis by osteoblasts during bone development and homeostasis. Front Endocrinol (Lausanne). 2013;4:85.
Jaakkola P, Mole D, Tian Y, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72.
Bruick R, McKnight S. A conserved family of prolyl-4-hydroxilases that modify HIF. Science. 2002;294.
Maxwell P, Wiesener M, Chang G, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5.
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408.
Schipani E, Ryan HE, Didrickson S, et al. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–76.
Wang Y, Wan C, Deng L, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–26.
Tavassoli M, Crosby WH. Transplantation of marrow to extramedullary sites. Science. 1968;161:54–6.
Kaback LA, Soungdo Y, Naik A, et al. Osterix/Sp7 regulates mesenchymal stem cell mediated endochondral ossification. J Cell Physiol. 2008;214:173–82.
Deguchi K, Yagi H, Inada M, et al. Excessive extramedullary hematopoiesis in Cbfa1-deficient mice with a congenital lack of bone marrow. Biochem Biophys Res Commun. 1999;255:352–9.
Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–64.
Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105:16976–81.
Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–12.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34.
Rankin EB, Wu C, Khatri R, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149:63–74. This paper is the first to demonstrate that cells of the osteoblastic lineage have the capacity to produce EPO under physiologic and pathophysiologic conditions.
Elliott S, Sinclair AM. The effect of erythropoietin on normal and neoplastic cells. Biogeosciences. 2012;6:163–89.
Obara N, Suzuki N, Kim K, et al. Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood. 2008;111:5223–32.
Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–9. This paper demonstrates that Osterix positive cells in the bone marrow give rise to primitive and definitive stromal cells as well as cells of the osteoblastic lineage.
Kertesz N, Wu J, Chen TH, et al. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276:101–10.
Wu C, Rankin EB, Giaccia AJ. Blood and bones: osteoblastic HIF signaling regulates erythropoiesis. Cell Cycle. 2012;11:2221–2.
Lucas TS, Bab IA, Lian JB, et al. Stimulation of systemic bone formation induced by experimental blood loss. Clin Orthop Relat Res. 1997;267:75.
Wan L, Zhang F, He Q, et al. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS One. 2014;9:e102010.
Garcia P, Speidel V, Scheuer C, et al. Low dose erythropoietin stimulates bone healing in mice. J Orthop Res. 2011;29:165–72.
Holstein JH, Orth M, Scheuer C, et al. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone. 2011;49:1037–45.
Sun H, Jung Y, Shiozawa Y, et al. Erythropoietin modulates the structure of bone morphogenetic protein 2-engineered cranial bone. Tissue Eng Part A. 2012;18:2095–105.
Nair AM, Tsai YT, Shah KM, et al. The effect of erythropoietin on autologous stem cell-mediated bone regeneration. Biomaterials. 2013;34:7364–71.
Rolfing JH, Bendtsen M, Jensen J, et al. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J Orthop Res. 2012;30:1083–8.
Betsch M, Thelen S, Santak L, et al. The role of erythropoietin and bone marrow concentrate in the treatment of osteochondral defects in mini-pigs. PLoS One. 2014;9:e92766.
Rolfing JH, Jensen J, Jensen JN, et al. A single topical dose of erythropoietin applied on a collagen carrier enhances calvarial bone healing in pigs. Acta Orthop. 2014;85:201–9.
Shiozawa Y, Jung Y, Ziegler AM, et al. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5:e10853.
Wu H, Lee SH, Gao J, et al. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–605.
Rolfing JH, Baatrup A, Stiehler M, et al. The osteogenic effect of erythropoietin on human mesenchymal stromal cells is dose-dependent and involves non-hematopoietic receptors and multiple intracellular signaling pathways. Stem Cell Rev. 2014;10:69–78.
Kim J, Jung Y, Sun H, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113:220–8.
Elliott S, Sinclair A, Collins H, et al. Progress in detecting cell-surface protein receptors: the erythropoietin receptor example. Ann Hematol. 2014;93:181–92.
Singbrant S, Russell MR, Jovic T, et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117:5631–42.
Compliance with Ethics Guidelines
Conflict of Interest
C. Wu, A. J. Giaccia, and E. B. Rankin declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by C. Wu, A. J. Giaccia, and E. B. Rankin involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, C., Giaccia, A.J. & Rankin, E.B. Osteoblasts: a Novel Source of Erythropoietin. Curr Osteoporos Rep 12, 428–432 (2014). https://doi.org/10.1007/s11914-014-0236-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-014-0236-x