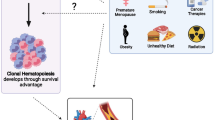
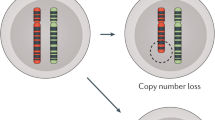
Abstract
Purpose of Review
Clonal hematopoiesis (CH) is an age-dependent process detectable using advanced sequencing technologies and is associated with multiple adverse health outcomes including cardiovascular disease and cancer. The purpose of this review is to summarize known causes of CH mutations and to identify key areas and considerations for future research on CH.
Recent Findings
Studies have identified multiple potential causes of CH mutations including smoking, cancer therapies, cardiometabolic disease, inflammation, and germline risk factors. Additionally, large-scale studies have facilitated the identification of gene-specific effects of CH mutation risk factors that may have unique downstream health implications. For example, cancer therapies and sources of environmental radiation appear to cause CH through their impact on DNA damage repair genes.
Summary
There is a growing body of evidence defining risk factors for CH mutations. Standardization in the identification of CH mutations may have important implications for future research. Additional studies in underrepresented populations and their diverse environmental exposures are needed to facilitate broad public health impact of the study of CH mutations.

Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366(6465):eaan4673.
Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74(4):567–77.
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16.
Evans MA, Sano S, Walsh K. Cardiovascular disease, aging, and clonal hematopoiesis. Annu Rev Pathol. 2020;15:419–38.
Gibson CJ, Steensma DP. New insights from studies of clonal hematopoiesis. Clin Cancer Res. 2018;24(19):4633–42.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98.
Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–87.
Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–8.
McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 2015;10(8):1239–45.
Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559(7714):400–4.
• Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52(11):1219–26. MSK-IMPACT study showed that cancer therapy increased the CH mutation frequency in DNA damage repair response (DDR) genes including TP53, PPM1D, CHEK2, and ATM, which also increased the risk of secondary cancers, called therapy-related myeloid neoplasms.
Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A. Role of p53 in the Regulation of cellular senescence. Biomolecules. 2020;10(3):420.
• Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Bao EL, Zekavat SM, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586(7831):763–8. Nearly 100,000 whole genomes from the NHLBI TOP Med project were used for replicating the association of germline variants in TERT with CHIP and demonstrating novel genetic loci spanning KPNA4 and TRIM59 and near TET2, and rs14441806, African ancestry specific intergenic variant near TET2, which are associated with DNMT3A and TET2 CH mutations.
• Kar SP, Quiros PM, Gu M, Jiang T, Mitchell J, Langdon R, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet. 2022;54(8):1155–66. Whole-exome sequencing data from over 200,000 samples of the UK Biobank identified new genetic loci associated with CH in PARP1, ATM, CHEK2, CD164, SETBP1. Showed age-dependent association patterns with DNMT3A clones associated with TCL1A and CD164 germline variants expanding faster in early life and TET2 clones associated with TERT germline variants expanding at a constant rate over the lifetime.
Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130(6):742–52.
Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78.
Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374-82.e4.
Pich O, Reyes-Salazar I, Gonzalez-Perez A, Lopez-Bigas N. Discovering the drivers of clonal hematopoiesis. Nat Commun. 2022;13(1):4267.
Buscarlet M, Provost S, Zada YF, Barhdadi A, Bourgoin V, Lepine G, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130(6):753–62.
Walter MJ, Shen D, Shao J, Ding L, White BS, Kandoth C, et al. Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia. 2013;27(6):1275–82.
Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496–506.
Young AL, Tong RS, Birmann BM, Druley TE. Clonal hematopoiesis and risk of acute myeloid leukemia. Haematologica. 2019;104(12):2410–7.
Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152(4):714–26.
Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373(1):35–47.
Babushok DV, Olson TS, Bessler M. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373(17):1673.
Busque L, Buscarlet M, Mollica L, Levine RL. Concise review: age-related clonal hematopoiesis: stem cells tempting the devil. Stem Cells. 2018;36(9):1287–94.
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377(2):111–21.
Bhattacharya R, Zekavat SM, Haessler J, Fornage M, Raffield L, Uddin MM, et al. Clonal hematopoiesis is associated with higher risk of stroke. Stroke. 2022;53(3):788–97.
Fuster JJ, Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res. 2018;122(3):523–32.
Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98–105.
• Nowakowska MK, Kim T, Thompson MT, Bolton KL, Deswal A, Lin SH, et al. Association of clonal hematopoiesis mutations with clinical outcomes: a systematic review and meta-analysis. Am J Hematol. 2022;97(4):411–20. The meta-analysis of 32 studies verified the clinical significance of CH mutations for cardiovascular diseases, hematologic malignancies, therapy-related myeloid neoplasms, and death. Studies reporting on CH mutations with a variant allele fraction ≥ 10% showed the strongest association with adverse health outcomes.
Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–7.
Miller PG, Gibson CJ, Mehta A, Sperling AS, Frederick DT, Manos MP, et al. Fitness landscape of clonal hematopoiesis under selective pressure of immune checkpoint blockade. JCO Precis Oncol. 2020;4:1027–1033.
Mitchell E, Spencer Chapman M, Williams N, Dawson KJ, Mende N, Calderbank EF, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature. 2022;606(7913):343–50.
• Watson CJ, Papula AL, Poon GYP, Wong WH, Young AL, Druley TE, et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science. 2020;367(6485):1449–54. Evolutionary selection and variant density by variant allele frequency were modeled by branching process from exisitng epidemiological clonal hematopoiesis stuides.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448(7155):767–74.
Nakao T, Bick AG, Taub MA, Zekavat SM, Uddin MM, Niroula A, et al. Mendelian randomization supports bidirectional causality between telomere length and clonal hematopoiesis of indeterminate potential. Sci Adv. 2022;8(14):eab6l579.
• Fabre MA, de Almeida JG, Fiorillo E, Mitchell E, Damaskou A, Rak J, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606(7913):335–42. Conducted logitudinal exponential growth modeling and phylogenetic analysis to establish the natural histroy of clonal hematopoiesis.
Levin MG, Nakao T, Zekavat SM, Koyama S, Bick AG, Niroula A, et al. Genetics of smoking and risk of clonal hematopoiesis. Sci Rep. 2022;12(1):7248.
Cheng S, Zhang W, Inghirami G, Tam W. Mutation analysis links angioimmunoblastic T-cell lymphoma to clonal hematopoiesis and smoking. Elife. 2021;10:e66395.
Dawoud AAZ, Tapper WJ, Cross NCP. Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia. 2020;34(10):2660–72.
Kessler MD, Damask A, O’Keeffe S, Van Meter M, Banerjee N, Semrau S, et al. Exome sequencing of 628,388 individuals identifies common and rare variant associations with clonal hematopoiesis phenotypes. medRxiv. 2022:2021.12.29.21268342.
Wong TN, Miller CA, Jotte MRM, Bagegni N, Baty JD, Schmidt AP, et al. Cellular stressors contribute to the expansion of hematopoietic clones of varying leukemic potential. Nat Commun. 2018;9(1):455.
Gibson CJ, Lindsley RC, Tchekmedyian V, Mar BG, Shi J, Jaiswal S, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35(14):1598–605.
Hsu JI, Dayaram T, Tovy A, De Braekeleer E, Jeong M, Wang F, et al. PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell. 2018;23(5):700-13.e6.
Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125(9):1367–76.
Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518(7540):552–5.
Haring B, Reiner AP, Liu JM, Tobias DK, Whitsel E, Berger JS, et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the Women's Health Initiative. J Am Heart Assoc. 2021;10(5):e018789.
Bonnefond A, Skrobek B, Lobbens S, Eury E, Thuillier D, Cauchi S, et al. Association between large detectable clonal mosaicism and type 2 diabetes with vascular complications. Nat Genet. 2013;45(9):1040–3.
Florez MA, Tran BT, Wathan TK, DeGregori J, Pietras EM, King KY. Clonal hematopoiesis: mutation-specific adaptation to environmental change. Cell Stem Cell. 2022;29(6):882–904.
Wilkinson AC, Yamazaki S. The hematopoietic stem cell diet. Int J Hematol. 2018;107(6):634–41.
Cai Z, Lu X, Zhang C, Nelanuthala S, Aguilera F, Hadley A, et al. Hyperglycemia cooperates with Tet2 heterozygosity to induce leukemia driven by proinflammatory cytokine-induced lncRNA Morrbid. J Clin Invest. 2021;131(1):e140707.
Cabezas-Wallscheid N, Buettner F, Sommerkamp P, Klimmeck D, Ladel L, Thalheimer FB, et al. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell. 2017;169(5):807-23.e19.
Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549(7673):476–81.
Chen JQ, Nie DJ, Wang XY, Wang L, Wang F, Zhang Y, et al. Enriched clonal hematopoiesis in seniors with dietary vitamin C inadequacy. Clin Nutr Espen. 2021;46:179–84.
Cortes M, Chen MJ, Stachura DL, Liu SY, Kwan W, Wright F, et al. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016;17(2):458–68.
Studzinski GP, Harrison JS, Wang X, Sarkar S, Kalia V, Danilenko M. Vitamin D control of hematopoietic cell differentiation and leukemia. J Cell Biochem. 2015;116(8):1500–12.
Weber S, Parmon A, Kurrle N, Schnutgen F, Serve H. The clinical significance of iron overload and iron metabolism in myelodysplastic syndrome and acute myeloid leukemia. Front Immunol. 2021;11:627662.
Bondu S, Alary AS, Lefevre C, Houy A, Jung G, Lefebvre T, et al. A variant erythroferrone disrupts iron homeostasis in SF3B1-mutated myelodysplastic syndrome. Sci Transl Med. 2019;11(500):eaav5467.
Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130(15):1693–8.
King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11(10):685–92.
King KY, Huang Y, Nakada D, Goodell MA. Environmental influences on clonal hematopoiesis. Exp Hematol. 2020;83:66–73.
Bick AG, Popadin K, Thorball CW, Uddin MM, Zanni MV, Yu B, et al. Increased prevalence of clonal hematopoiesis of indeterminate potential amongst people living with HIV. Sci Rep. 2022;12(1):577.
Dharan NJ, Yeh P, Bloch M, Yeung MM, Baker D, Guinto J, et al. HIV is associated with an increased risk of age-related clonal hematopoiesis among older adults. Nat Med. 2021;27(6):1006–11.
Zhang CRC, Nix D, Gregory M, Ciorba MA, Ostrander EL, Newberry RD, et al. Inflammatory cytokines promote clonal hematopoiesis with specific mutations in ulcerative colitis patients. Exp Hematol. 2019;80:36–41.
Kristinsson SY, Bjorkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897–903.
Hormaechea-Agulla D, Matatall KA, Le DT, Kain B, Long XC, Kus P, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFN gamma signaling. Cell Stem Cell. 2021;28(8):1428–42.
Matatall KA, Jeong MR, Chen SY, Sun DQ, Chen FJ, Mo QX, et al. Chronic infection depletes hematopoietic stem cells through stress-induced terminal differentiation. Cell Rep. 2016;17(10):2584–95.
Cai ZG, Kotzin JJ, Ramdas B, Chen SS, Nelanuthala S, Palam LR, et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell. 2018;23(6):833–49.
Abegunde SO, Buckstein R, Wells RA, Rauh MJ. An inflammatory environment containing TNFalpha favors Tet2-mutant clonal hematopoiesis. Exp Hematol. 2018;59:60–5.
Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 2018;557(7706):580–4.
Jasra S, Giricz O, Zeig-Owens R, Pradhan K, Goldfarb DG, Barreto-Galvez A, et al. High burden of clonal hematopoiesis in first responders exposed to the World Trade Center disaster. Nat Med. 2022;28(3):468–71.
Brojakowska A, Kour A, Thel MC, Park E, Bisserier M, Garikipati VNS, et al. Retrospective analysis of somatic mutations and clonal hematopoiesis in astronauts. Commun Biol. 2022;5(1):828.
Mencia-Trinchant N, MacKay MJ, Chin C, Afshinnekoo E, Foox J, Meydan C, et al. Clonal hematopoiesis before, during, and after human spaceflight (vol 33, 108458, 2020). Cell Reports. 2021;34(6):108458.
Hinds DA, Barnholt KE, Mesa RA, Kiefer AK, Do CB, Eriksson N, et al. Germline variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood. 2016;128(8):1121–8.
Tajuddin SM, Schick UM, Eicher JD, Chami N, Giri A, Brody JA, et al. Large-scale exome-wide association analysis identifies loci for white blood cell traits and pleiotropy with immune-mediated diseases. Am J Hum Genet. 2016;99(1):22–39.
Jaiswal S. Clonal hematopoiesis and nonhematologic disorders. Blood. 2020;136(14):1606–14.
Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484.
Fox EJ, Reid-Bayliss KS, Emond MJ, Loeb LA. Accuracy of next generation sequencing platforms. Next Gener Seq Appl. 2014;11000106.
Spencer DH, Tyagi M, Vallania F, Bredemeyer AJ, Pfeifer JD, Mitra RD, et al. Performance of common analysis methods for detecting low-frequency single nucleotide variants in targeted next-generation sequence data. J Mol Diagn. 2014;16(1):75–88.
van Zeventer IA, Salzbrunn JB, de Graaf AO, van der Reijden BA, Boezen HM, Vonk JM, et al. Prevalence, predictors, and outcomes of clonal hematopoiesis in individuals aged >/=80 years. Blood Adv. 2021;5(8):2115–22.
Zioni N, Chapal Ilani N, Petrovich-Kopitman E, Saçma M, Geiger H, Scheller M, et al. Fatty bone marrow positively selects pre-leukemic HSPCs with a DNMT3A-mutation. Blood. 2021;138(Supplement 1):596-.
Kahn JD, Miller PG, Silver AJ, Sellar RS, Bhatt S, Gibson C, et al. PPM1D-truncating mutations confer resistance to chemotherapy and sensitivity to PPM1D inhibition in hematopoietic cells. Blood. 2018;132(11):1095–105.
Yura Y, Miura-Yura E, Katanasaka Y, Min KD, Chavkin N, Polizio AH, et al. The cancer therapy-related clonal hematopoiesis driver gene Ppm1d promotes inflammation and non-ischemic heart failure in mice. Circ Res. 2021;129(6):684–98.
Kim B, Won D, Lee ST, Choi JR. Somatic mosaic truncating mutations of PPM1D in blood can result from expansion of a mutant clone under selective pressure of chemotherapy. Plos One. 2019;14(6):e0217521.
Voso MT, Pandzic T, Falconi G, Dencic-Fekete M, De Bellis E, Scarfo L, et al. Clonal haematopoiesis as a risk factor for therapy-related myeloid neoplasms in patients with chronic lymphocytic leukaemia treated with chemo-(immuno)therapy. Br J Haematol. 2022;198(1):103–13.
Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559(7715):637–41.
Acknowledgements
We thank Ms. Sarah Bronson, ELS, from The University of Texas MD Anderson Cancer Center Research Medical Library, for her expertise in scientific manuscript editing.
Funding
This research was supported, in part, by Cancer Center Support Grant P30 CA016672 from the National Institutes of Health. Dr. Nead is a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar in Cancer Research. Dr Nead is supported by grant RR190077 from CPRIT and grants L30CA253796 and K08CA263313 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
LiJin Joo, Catherine C. Bradley, Paul A. Scheet, and Kevin T. Nead declare no conflict of interest. Steven H. Lin has received grants or contracts from STCube Pharmaceuticals, Beyond Spring Pharmaceuticals, and Nektar Therapeutics; consulting fees from XRAD Therapeutics; and has participated on a Data Safety Monitoring or Advisory Boards for Creatv Microtech and AstraZeneca.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Leukemia
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joo, L., Bradley, C.C., Lin, S.H. et al. Causes of Clonal Hematopoiesis: a Review. Curr Oncol Rep 25, 211–220 (2023). https://doi.org/10.1007/s11912-023-01362-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01362-z