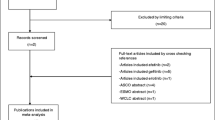
Abstract
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have been extensively investigated in previously treated advanced non-small-cell lung cancer (NSCLC), but what it is still unclear is the efficacy of (EFGR-TKIs: gefitinib or erlotinib) monotherapy in previously treated non-small-cell lung cancer (NSCLC). In December 2013, we performed a search in the PubMed, EMBASE, Cochrane library databases and Web of Science for randomized trials exploring the role of gefitinib or erlotinib in advanced non-small cell lung cancer. Through strict inclusion and exclusion criteria, fourteen trials (three front-line, two second-line, nine maintenance, n = 8970 patients) were eligible. EGFR-TKIs significantly increased overall survival (OS) [hazard ratio (HR) 0.88, 95 %confidence interval (CI) 0.82–0.96, I 2 = 50.5 %] and progression-free survival (PFS) (HR 0.71, 95 % CI 0.63–0.81, I 2 = 81.2 %] compared with placebo or best support care (BSC). Patients with clinical features such as never smoker, adenocarcinoma, Asian ethnicity and EGFR mutation positive had more pronounced OS and PFS benefit. The main adverse reactions were diarrhea, rashes, anorexia and anemia, [odds ratio (OR) = 3.635, 95 % confidence interval (CI) = (2.377 to 5.557)], [OR = 15.664, 95 %CI = (8.869 to 27.665)], [OR = 1.555, 95 %CI = (1.060 to 2.283)], [OR = 1.481, 95 %CI = (1.114 to 1.969)], respectively. The results show that monotherapy therapy with EFGR-TKIs produce a significant OS and PFS benefit for patients with NSCLC compared with placebo or BSC, especially for the patients who had adenocarcinomas, non-smokers and patients with EGFR gene mutations.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics,2011. CA: a cancer journal for clinicians. 2011;61(2):69–90.
Lee SM, Khan I, Upadhyay S, Lewanski C, Falk S, Skailes G, et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. The lancet oncology. 2012;13(11):1161–70. doi:10.1016/S1470-2045(12)70412-6. This RCT showed first-line treatment with erlotinib did not improve overall survival in all unselected patients with advanced NSCLC deemed unsuitable for chemotherapy treatment, who usually have a poor prognosis (about 3–4 months median overall survival). However, erlotinib did improve progression-free survival. Additionally, erlotinib significantly improved both overall survival and progression-free survival for patients who developed a first-cycle rash.
Tang X, Shigematsu H, Bekele BN, Roth JA, Minna JD, Hong WK, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65(17):7568–72.
Sato M, Shames DS, Gazdar AF, Minna JD. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol. 2007;2(4):327–43.
Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7(10):778–90.
Alimujiang S, Zhang T, Han ZG, Yuan SF, Wang Q, Yu TT, et al. Epidermal growth factor receptor tyrosine kinase inhibitor versus placebo as maintenance therapy for advanced non- small-cell lung cancer: a meta-analysis of randomized controlled trials. Asian Pacific journal of cancer prevention : APJCP. 2013;14(4):2413–9. This overview of several RCTs demonstrated that EGFR-TKIs demonstrated encouraging efficacy, safety and survival when delivered as maintenance therapy for patients with advanced NSCLC after first-line chemotherapy, especially for the patients who had adenocarcinomas, were female, non-smokers and patients with EGFR gene mutations.
Chen X, Liu Y, Roe OD, Qian Y, Guo R, Zhu L, et al. Gefitinib or erlotinib as maintenance therapy in patients with advanced stage non-small cell lung cancer: a systematic review. PLoS One. 2013;8(3):e59314. doi:10.1371/journal.pone.0059314. This overview of several RCTs demonstrated that maintenance therapy with erlotinib or gefitinib produces a significant PFS and OS benefit for unselected patients with advanced NSCLC compared with placebo or observation. Given the less toxicity of TKIs than chemotherapy and simple oral administration, this treatment strategy seems to be of important clinical value.
Qi W-X, Wang Q, Jiang Y-L, Sun Y-J, Tang L-n, He A-n et al. Overall survival benefits for combining targeted therapy as second-line treatment for advanced non-small-cell-lung cancer: a meta-analysis of published data. PloS one. 2013;8(2). doi:10.1371/journal.pone.0055637. This overview of several RCTs demonstrated that combining targeted therapy seems superior over erlotinib monotherapy as second-line treatment for advanced NSCLC.
Qi W-X, Shen Z, Lin F, Sun Y-J, Min D-L, Tang L-N, et al. Comparison of the Efficacy and Safety of EFGR Tyrosine Kinase Inhibitor Monotherapy with Standard Second-line Chemotherapy in Previously Treated Advanced Non-small-cell Lung Cancer: a Systematic Review and Meta-analysis. Asian Pacific Journal of Cancer Prevention. 2012;13(10):5177–82. doi:10.7314/apjcp.2012.13.10.5177. This overview of several RCTs demonstrated that EGFR-TKI monotherapy tended to be more effective in East Asian patients in terms of PFS and ORR compared with standard second-line chemotherapy.
Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105(9):595–605. doi:10.1093/jnci/djt072. This overview of a large number of RCTs demonstrated that EGFR-TKIs therapy statistically significantly delays disease progression in EGFRmut + patients but has no demonstrable impact on OS. EGFR mutation is a predictive biomarker of PFS benefit with EGFR-TKIs treatment in all settings. This study and our study have certain similarities, so we refer to some of its methods and data.
Goss G, Ferry D, Wierzbicki R, Laurie SA, Thompson J, Biesma B, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(13):2253–60. doi:10.1200/jco.2008.18.4408.
Goss GD, O'Callaghan C, Lorimer I, Tsao MS, Masters GA, Jett J, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(27):3320–6. doi:10.1200/jco.2013.51.1816.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32. doi:10.1056/NEJMoa050753.
Gaafar RM, Surmont VF, Scagliotti GV, Van Klaveren RJ, Papamichael D, Welch JJ, et al. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03). Eur J Cancer. 2011;47(15):2331–40. doi:10.1016/j.ejca.2011.06.045. This RCT showed gefitinib is safe and improves PFS. However, no difference in OS was observed in this study.
Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non–small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25(12):1545–52.
Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non–small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–9.
Mok TS, Wu YL, Yu CJ, Zhou C, Chen YM, Zhang L, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(30):5080–7. doi:10.1200/jco.2008.21.5541.
Pérol M, Chouaid C, Pérol D, Barlési F, Gervais R, Westeel V, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non–small-cell lung cancer. J Clin Oncol. 2012;30(28):3516–24. This RCT showed gemcitabine continuation maintenance or erlotinib switch maintenance significantly reduces disease progression in patients with advanced NSCLC treated with cisplatin gemcitabine as first-line chemotherapy. Response to induction chemotherapy may affect OS only for continuation maintenance.
Zhang L, Ma S, Song X, Han B, Cheng Y, Huang C, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. The lancet oncology. 2012;13(5):466–75. doi:10.1016/S1470-2045(12)70117-1. This RCT showed maintenance treatment with gefitinib significantly prolonged progression-free survival compared with placebo in patients from East Asia with advanced NSCLC who achieved disease control after first-line chemotherapy. Clinicians should consider these data when making decisions about maintenance treatment in such patients.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi:10.1002/sim.1186.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi:10.1016/0197-2456(86)90046-2.
Petitti DB. Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. USA: Oxford University Press; 1999.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. doi:10.2307/2533446.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366(9496):1527–37. doi:10.1016/S0140-6736(05)67625-8.
Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. The lancet oncology. 2010;11(6):521–9. doi:10.1016/S1470-2045(10)70112-1.
Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non–small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26(15):2450–6.
Wu YL, Kim JH, Park K, Zaatar A, Klingelschmitt G, Ng C. Efficacy and safety of maintenance erlotinib in Asian patients with advanced non-small-cell lung cancer: a subanalysis of the phase III, randomized SATURN study. Lung Cancer. 2012;77(2):339–45. doi:10.1016/j.lungcan.2012.03.012.
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–74. This RCT showed erlotinib was effective and well tolerated in Asian patients, producing benefits consistent with those observed in the overall SATURN population. Maintenance treatment with erlotinib appears to be a useful option for the management of Asian patients with advanced NSCLC without progression after first-line chemotherapy.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39.
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500.
Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–11.
Yang C-H. EGFR tyrosine kinase inhibitors for the treatment of NSCLC in East Asia: present and future. Lung Cancer. 2008;60:S23–30.
Jiang J, Huang L, Liang X, Zhou X, Huang R, Chu Z, et al. Gefitinib versus docetaxel in previously treated advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Acta Oncol. 2011;50(4):582–8.
Compliance with Ethics Guidelines
Conflict of Interest
XiongWen Yang, Ke Yang, and KangYu Kuang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lung Cancer
Rights and permissions
About this article
Cite this article
Yang, X., Yang, K. & Kuang, K. The Efficacy and Safety of EGFR Inhibitor Monotherapy in Non-Small Cell Lung Cancer: A Systematic Review. Curr Oncol Rep 16, 390 (2014). https://doi.org/10.1007/s11912-014-0390-4
Published:
DOI: https://doi.org/10.1007/s11912-014-0390-4